AOD-9604

AOD-9604
Commonly researched with these items
Patent-pending lot tracking: Complete transparency from production to vial.
AOD-9604: Growth Hormone Fragment for Metabolic Research Applications
Disclaimer
Products described here are supplied for research use only and are not intended for diagnostic, therapeutic, or clinical application. All statements regarding biological activity reflect preclinical and in vitro findings exclusively and have not been evaluated by the U.S. Food and Drug Administration. These materials and related content are provided for educational and investigational purposes only and are intended solely for qualified researchers in laboratory and academic settings.
Introduction: From Growth Hormone Discovery to Targeted Research Applications
AOD-9604 represents a sophisticated breakthrough in peptide research, emerging from innovative investigations at Monash University that revolutionized our understanding of growth hormone's metabolic effects. When researchers discovered that the fat-reducing properties of HGH could be isolated to a specific 16-amino acid fragment representing less than 10% of the total molecule, they unlocked new possibilities for targeted metabolic research. This synthetic peptide, derived from the C-terminal region of mammalian growth hormone (amino acids 176-191), was developed by Metabolic Pharmaceuticals Ltd. during the early 2000s as a research tool for investigating cellular energy regulation and fat metabolism without the broader physiological effects associated with full-length growth hormone.
Laboratory investigations have revealed AOD-9604's unique capacity to stimulate lipolysis through β3-adrenergic receptor modulation while maintaining complete independence from growth hormone receptor pathways. This selective mechanism enables researchers to study fat oxidation and metabolic regulation without the confounding effects of IGF-1 elevation, hyperglycemia, or insulin resistance that characterize traditional growth hormone research. The peptide's sophisticated molecular engineering, including strategic placement of a tyrosine residue at the N-terminus and stabilization through disulfide bridge formation, demonstrates advanced peptide design principles that enhance both stability and research utility.
Contemporary research applications extend far beyond the original metabolic focus to include tissue regeneration studies, analytical method development, and cellular energy pathway investigations. Laboratory models demonstrate AOD-9604's exceptional safety profile, with comprehensive toxicology studies providing researchers with confidence in experimental protocols. The compound's well-characterized pharmacokinetics, defined metabolic pathways, and extensive safety database position AOD-9604 as an invaluable research tool for advancing our understanding of metabolic regulation, receptor signaling, and cellular energy homeostasis in laboratory and academic settings.
Molecular Structure and Engineering Principles
Research investigations reveal AOD-9604's sophisticated molecular architecture, designed through advanced peptide engineering principles to maximize stability and biological activity in experimental settings. The peptide consists of a 16-amino acid sequence (H-Tyr-Leu-Arg-Ile-Val-Gln-Cys-Arg-Ser-Val-Glu-Gly-Ser-Cys-Gly-Phe-OH) with a molecular mass of 1813.8604 Da, derived from the C-terminal region of mammalian growth hormone (amino acids 176-191). Laboratory studies demonstrate that the strategic addition of a tyrosine residue at the N-terminus enhances peptide stability and facilitates analytical detection, while the formation of a disulfide bridge between cysteine residues at positions 7 and 14 provides crucial structural stabilization under experimental conditions.
Molecular stability studies demonstrate AOD-9604's resistance to degradation under extreme pH conditions and exposure to digestive enzymes, making it particularly valuable for oral administration protocols in animal research models. The peptide's engineered structure maintains biological activity across diverse experimental conditions, enabling researchers to explore various administration routes and dosing protocols without concerns about rapid degradation. Laboratory investigations show that the disulfide bridge formation creates a stable cyclic structure that preserves the peptide's active conformation while the terminal modifications enhance both stability and bioavailability in research applications.
Structural comparison studies reveal how AOD-9604's design principles represent advanced understanding of peptide pharmacology and research development. Research demonstrates that the fragment retains the essential lipolytic domain of growth hormone while eliminating sequences responsible for growth-promoting and hyperglycemic effects, providing researchers with a targeted tool for studying specific metabolic pathways. This molecular precision enables investigation of β3-adrenergic receptor signaling without the complex interactions associated with full-length hormone studies, offering researchers unprecedented specificity in metabolic research applications and cellular pathway investigations.
β3-Adrenergic Receptor Mechanisms and Lipolytic Pathways
Laboratory research demonstrates AOD-9604's primary mechanism centers on β3-adrenergic receptor (β3-AR) modulation, establishing it as a powerful tool for investigating cellular energy regulation and fat metabolism in experimental models. Studies by Ng et al. reveal that AOD-9604 increases β3-AR mRNA expression in adipose tissue, restoring receptor levels in obese animal models to those comparable with lean controls. This restoration of receptor expression provides researchers with insights into the relationship between receptor density and metabolic function, while offering a mechanism for studying cellular energy regulation in compromised metabolic states.
Mechanistic validation studies using β3-AR knockout mice provide definitive evidence of pathway specificity, with research demonstrating that long-term AOD-9604 administration fails to produce weight changes in knockout animals while maintaining effectiveness in wild-type controls. This genetic validation confirms the peptide's dependence on β3-AR signaling for chronic metabolic effects, providing researchers with confidence in pathway-specific studies and enabling investigation of receptor-dependent mechanisms in various experimental models. Laboratory protocols utilizing this knockout model system enable researchers to distinguish between β3-AR-dependent and independent effects in metabolic research applications.
The lipolytic cascade activated by AOD-9604 follows the established pathway: β3-AR activation → cAMP elevation → hormone-sensitive lipase activation → triglyceride breakdown → free fatty acids and glycerol release. Research protocols demonstrate increased plasma glycerol levels as a biomarker of enhanced lipolysis, providing researchers with quantitative measures of peptide activity in experimental settings. Laboratory studies show that this mechanism operates independently of food consumption patterns, enabling researchers to study pure metabolic effects without confounding variables related to appetite or feeding behavior, making AOD-9604 particularly valuable for controlled metabolic investigations and energy regulation studies.
Growth Hormone Receptor Independence and Metabolic Selectivity
Research investigations establish AOD-9604's critical independence from growth hormone receptors, providing researchers with a unique tool for studying targeted metabolic effects without the complex physiological changes associated with traditional growth hormone research. Laboratory studies demonstrate that AOD-9604 does not compete for growth hormone receptors and operates through novel pathways distinct from hGH-stimulated mechanisms, enabling researchers to investigate lipolytic pathways in isolation. This receptor independence explains the absence of IGF-1 elevation, hyperglycemic effects, cell proliferation stimulation, and insulin resistance development that characterize full-length growth hormone studies.
Comparative research protocols demonstrate how AOD-9604's selectivity enables sophisticated experimental designs where metabolic effects can be studied without confounding growth and anabolic responses. Laboratory models show that while both HGH and AOD-9604 reduce body weight and body fat in experimental animals, AOD-9604 avoids hGH-associated hyperglycemia and insulin secretion reduction, providing researchers with cleaner metabolic models for studying fat oxidation and energy expenditure. This selectivity is particularly valuable for researchers investigating metabolic disorders where growth hormone's broader effects would complicate experimental interpretation.
Advanced mechanistic studies reveal that AOD-9604's independence from growth hormone signaling pathways allows researchers to study metabolic regulation in aging models and growth hormone-deficient systems without introducing growth-related variables. Laboratory investigations demonstrate that the peptide maintains its lipolytic activity in models where growth hormone signaling is compromised, providing researchers with tools for studying metabolic function across diverse physiological conditions. This pathway independence enables investigation of metabolic regulation mechanisms that operate separately from traditional growth factor signaling, offering insights into alternative approaches for metabolic research and cellular energy regulation studies.
Laboratory Model Applications and Research Protocols
Extensive animal model research demonstrates AOD-9604's effectiveness across diverse experimental protocols, with Zucker rat studies establishing foundational dosing parameters and efficacy measures for metabolic research applications. Laboratory protocols utilizing 500 μg/kg body weight daily (oral administration) for 19 days in rodent models demonstrate alterations in body composition with reduced adipose tissue accumulation versus control groups, providing researchers with reproducible experimental frameworks for metabolic studies. Research investigations confirm increased lipolytic activity in adipose tissues without adverse effects on insulin sensitivity, as verified through euglycemic clamp techniques that enable precise metabolic assessments in experimental settings.
Chronic administration studies utilizing 14-day intraperitoneal protocols with mini-osmotic pumps provide researchers with sustained-release experimental models for investigating long-term metabolic effects. Laboratory research demonstrates modulation of lipid oxidation pathways and energy expenditure in experimental animals, with quantitative measures of metabolic rate changes providing researchers with objective endpoints for experimental protocols. These studies establish that AOD-9604's metabolic effects persist throughout chronic administration periods, enabling researchers to investigate both acute and sustained metabolic responses in various animal models and experimental conditions.
Advanced research applications include combination protocols where AOD-9604 serves as a metabolic primer in conjunction with other research compounds, providing insights into synergistic metabolic regulation strategies. Laboratory investigations explore the peptide's utility in metabolic disorder models, aging research, and cellular energy regulation studies, with protocols adapted for specific research objectives and experimental requirements. Research demonstrates that AOD-9604's consistent effects across diverse animal models enable comparative studies and meta-analytical approaches, while its excellent safety profile permits extended experimental protocols and chronic administration studies without safety concerns limiting research applications.
Preclinical Safety Profile and Research Considerations
AOD-9604 benefits from comprehensive preclinical safety databases from extensive animal model investigations, providing researchers with robust safety information for experimental protocol development. Preclinical research investigations utilized diverse dosing ranges across multiple species including rodent models, rabbits, and non-human primates, with study durations extending from acute to chronic administration protocols. These extensive animal model studies demonstrate favorable safety profiles across all dosing ranges and administration routes, providing researchers with confidence in experimental protocol safety and enabling exploration of diverse dosing strategies in laboratory settings.
Safety assessment protocols in animal models demonstrate favorable safety profiles across multiple species, with no anti-AOD9604 antibodies detected throughout chronic administration studies. Laboratory investigations show no significant changes in physiological parameters or clinical chemistry measures in research animal models, providing researchers with evidence of the peptide's excellent tolerance profile across diverse experimental conditions and species. Research protocols confirm maintenance of normal carbohydrate metabolism without glucose tolerance impairment or insulin resistance development in animal models, enabling researchers to study metabolic effects without concerns about metabolic complications or safety monitoring requirements.
Comprehensive toxicology studies including 6-month oral gavage studies in rats and 9-month protocols in cynomolgus monkeys provide researchers with extensive preclinical safety data supporting long-term experimental applications. Genotoxicity assessments including Ames tests, chromosomal aberration assays, and bone micronucleus assays demonstrate negative results across all testing paradigms, providing researchers with confidence in the peptide's genetic safety profile. This extensive safety database enables researchers to focus on scientific objectives rather than safety concerns, while the well-characterized safety profile facilitates regulatory approval for research protocols and institutional review board assessments for experimental studies.
Pharmacokinetic Profile and Analytical Methods
Research investigations reveal AOD-9604's distinct pharmacokinetic profile, characterized by rapid serum clearance with an approximate 4-minute half-life that provides researchers with precise temporal control over experimental exposures. Laboratory studies demonstrate amino-terminal truncation in cascade fashion as the primary degradation pathway, with six potential metabolites identified through advanced analytical methods. The most stable metabolite sequence (CRSVEGSCG) provides researchers with extended detection windows for analytical applications and enables investigation of metabolite activity in experimental protocols.
Advanced analytical methods developed for research applications include solid-phase extraction techniques for urine analysis with detection limits of 50 pg/mL, providing researchers with sensitive and specific analytical tools for pharmacokinetic studies and experimental monitoring. Research protocols demonstrate good linearity, precision (<20%), specificity, and recovery (62%) across diverse biological matrices, enabling quantitative assessment of peptide exposure in various experimental models. The parent compound becomes undetectable 56 minutes post-administration, while metabolite detection extends experimental monitoring capabilities for researchers investigating longer-term effects.
Analytical method development research provides valuable tools for studying peptide metabolism and degradation pathways in biological systems, with AOD-9604 serving as a model compound for peptide research applications. Laboratory protocols enable investigation of formulation effects, delivery system optimization, and bioavailability enhancement strategies, while the well-characterized degradation profile facilitates development of stabilized formulations for extended experimental protocols. Research applications include investigation of novel delivery systems, combination formulations, and sustained-release preparations that extend the peptide's research utility and enable investigation of chronic metabolic modulation effects in laboratory settings.
Novel Research Applications and Tissue Studies
Beyond traditional metabolic research, laboratory investigations demonstrate AOD-9604's potential in tissue regeneration studies, with research by Kwak et al. revealing significant applications in cartilage research using New Zealand white rabbit osteoarthritis models. Experimental protocols in rabbit models utilizing weekly intra-articular injections (0.25 mg AOD-9604 with or without 6 mg hyaluronic acid) for 4-7 weeks demonstrate alterations in cartilage metabolism markers when compounds are combined compared to individual administration, providing insights into potential synergistic mechanisms. This research expands AOD-9604's utility beyond metabolic applications and provides researchers with tools for investigating tissue repair mechanisms and combination research approaches.
Research investigations suggest AOD-9604's engagement in cellular repair mechanisms through fibroblast activity stimulation and extracellular matrix formation support, providing researchers with novel applications in tissue engineering and regenerative medicine studies. Laboratory models demonstrate the peptide's capacity to influence cellular repair pathways independently of its metabolic effects, enabling researchers to investigate tissue regeneration mechanisms and develop combination approaches for enhanced tissue repair. These findings provide foundation for expanded research applications in wound healing studies, tissue engineering protocols, and cellular regeneration investigations.
Advanced research applications include investigation of AOD-9604's role in cellular energy metabolism and mitochondrial function, with laboratory studies exploring how β3-adrenergic receptor signaling influences cellular energy production and utilization. Research protocols investigate the peptide's effects on cellular stress responses, metabolic adaptation mechanisms, and energy homeostasis under various experimental conditions. The compound's well-characterized safety profile and defined mechanisms enable researchers to explore novel applications while maintaining confidence in experimental safety and reproducibility, making AOD-9604 valuable for diverse research objectives in metabolic biology and cellular physiology.
Combination Research and Synergistic Applications
Laboratory research demonstrates AOD-9604's potential in combination protocols with other research compounds, providing insights into synergistic metabolic regulation strategies and multi-target research approaches. Research investigations explore combinations with hyaluronic acid for tissue regeneration applications, where the peptide's metabolic effects complement hyaluronic acid's structural support properties. Laboratory studies demonstrate enhanced outcomes in cartilage protection models when compounds are combined, providing researchers with frameworks for developing sophisticated combination approaches in tissue research and regenerative medicine applications.
Advanced research protocols investigate AOD-9604's utility as a metabolic primer in combination with other β3-adrenergic modulators, providing researchers with tools for studying receptor saturation effects and pathway interactions in metabolic regulation. Laboratory studies explore how the peptide's specific β3-AR modulation combines with other metabolic interventions to create synergistic effects in energy regulation and fat metabolism research. These combination approaches enable researchers to investigate complex metabolic networks and develop understanding of multi-pathway interactions in cellular energy regulation and metabolic homeostasis.
Research applications include investigation of AOD-9604 in combination with controlled activity protocols, dietary manipulation studies, and other metabolic modulators in animal models to understand how peptide-induced metabolic changes interact with physiological variables in experimental systems. Laboratory models enable researchers to study how β3-adrenergic activation influences responses to other metabolic stimuli, providing insights into integrative metabolism and adaptive responses in controlled research settings. The peptide's consistent effects and excellent safety profile enable complex experimental designs where multiple interventions can be studied simultaneously, advancing understanding of metabolic regulation and providing foundation for developing comprehensive approaches to metabolic research investigations.
Future Research Directions and Emerging Applications
Current research trajectories focus on expanding AOD-9604's applications beyond traditional metabolic studies to include investigation of cellular energy regulation, mitochondrial function, and metabolic adaptation mechanisms. Laboratory research explores how β3-adrenergic receptor signaling influences cellular stress responses, metabolic flexibility, and energy homeostasis under diverse experimental conditions. Research protocols investigate the peptide's potential in studying metabolic disorders, aging-related metabolic dysfunction, and cellular energy regulation mechanisms that extend beyond simple fat metabolism to encompass broader aspects of cellular physiology and metabolic health.
Advanced analytical method development continues to expand research capabilities, with ongoing investigations into novel detection methods, metabolite characterization, and biomarker development for studying peptide effects in complex biological systems. Research focuses on developing more sensitive analytical approaches that enable investigation of lower-dose effects and longer-term metabolic changes, while expanded metabolite profiling provides insights into peptide metabolism and cellular processing mechanisms. These analytical advances enable researchers to investigate subtle metabolic effects and develop understanding of peptide pharmacology in various experimental contexts.
Emerging research applications investigate AOD-9604's role in understanding fundamental questions about metabolic regulation, cellular energy homeostasis, and adaptive responses to metabolic challenges. Laboratory studies explore how targeted β3-adrenergic modulation influences cellular function beyond fat metabolism, including effects on inflammation, oxidative stress, and cellular survival mechanisms. Future research directions include investigation of personalized metabolic approaches utilizing AOD-9604 as a model compound for understanding individual variations in metabolic response patterns, while the peptide's well-characterized properties provide foundation for developing next-generation metabolic research tools and investigational approaches.
Research Protocol Considerations and Experimental Design
Laboratory protocol development for AOD-9604 research benefits from extensive pharmacokinetic and safety data from animal model studies that enable sophisticated experimental designs with confidence in reproducibility and safety. Preclinical research investigations have utilized various administration protocols in animal models for extended studies, while acute protocols may employ higher doses based on the extensive safety database from multiple species. The peptide's rapid clearance (4-minute half-life) enables precise temporal control over experimental exposures, while the well-characterized metabolite profile provides researchers with multiple analytical endpoints for comprehensive experimental assessment.
Experimental design considerations include the peptide's specific β3-adrenergic receptor dependence, which enables researchers to utilize β3-AR knockout models for mechanistic validation and pathway specificity confirmation. Laboratory protocols can incorporate genetic controls, receptor antagonists, and pathway inhibitors to dissect specific mechanisms and validate experimental findings. The compound's independence from growth hormone signaling enables clean experimental models where metabolic effects can be studied without confounding growth or anabolic responses, providing researchers with sophisticated tools for investigating isolated metabolic pathways.
Research applications require consideration of the peptide's rapid metabolism and clearance, with experimental protocols designed to account for short exposure windows or utilize sustained-release approaches for chronic studies. Laboratory studies demonstrate that oral administration requires higher doses but provides sustained exposure, while parenteral routes offer precise dosing control but require frequent administration for extended effects. The extensive toxicology database enables researchers to explore diverse dosing strategies and administration routes without safety concerns limiting experimental design, while the well-characterized effects provide foundation for power calculations and experimental planning in metabolic research applications.
Conclusion: Research Applications and Scientific Utility
AOD-9604 represents a sophisticated research tool that exemplifies advanced peptide engineering and targeted research development, providing researchers with unprecedented specificity for investigating metabolic regulation and cellular energy homeostasis. From its origins in growth hormone fragment research at Monash University to its current status as a well-characterized research compound with extensive safety documentation, AOD-9604 demonstrates how molecular engineering can create targeted tools for specific research applications. The peptide's unique combination of β3-adrenergic receptor specificity, growth hormone receptor independence, and excellent safety profile provides researchers with powerful capabilities for investigating metabolic pathways without the confounding effects associated with broader hormonal interventions.
Laboratory research has established AOD-9604 as an invaluable tool for investigating fundamental questions in metabolic biology, receptor signaling, and cellular energy regulation, with its well-characterized mechanisms and extensive safety database enabling researchers to focus on scientific objectives rather than safety concerns. The compound's applications extend beyond traditional metabolic research to include tissue regeneration studies, analytical method development, and combination research strategies that advance understanding of complex biological systems. Its consistent effects across diverse experimental models enable comparative studies and facilitate development of standardized research protocols for metabolic investigations.
Future research applications will likely expand to include investigation of metabolic adaptation mechanisms, cellular stress responses, and personalized metabolic approaches that utilize AOD-9604's well-characterized properties as foundation for understanding individual variations in metabolic regulation. The compound's role in advancing analytical method development, combination research strategies, and novel experimental approaches positions it at the forefront of metabolic research priorities. For researchers investigating cellular energy regulation, metabolic pathways, and receptor signaling mechanisms, AOD-9604 offers a sophisticated tool that combines mechanistic precision with practical utility, enabling advancement in our understanding of metabolic biology and the development of innovative approaches to metabolic research and intervention strategies.
References
- Heffernan, M.A., et al. (2000). Metabolic studies of a synthetic lipolytic domain (AOD9604) of human growth hormone. Journal of Endocrinology 167(3):493-504. https://doi.org/10.1677/joe.0.1670493
- Heffernan, M.A., et al. (2001). The effects of human GH and its lipolytic fragment (AOD9604) on lipid metabolism following chronic treatment in obese mice and beta(3)-AR knock-out mice. Endocrinology 142(12):5182-5189. https://doi.org/10.1210/endo.142.12.8522
- Heffernan, M.A., et al. (2001). Increase of fat oxidation and weight loss in obese mice caused by chronic treatment with human growth hormone or a modified C-terminal fragment. International Journal of Obesity 25(10):1442-1449. https://doi.org/10.1038/sj.ijo.0801740
- Heffernan, M.A., et al. (2000). Effects of oral administration of a synthetic fragment of human growth hormone on lipid metabolism. American Journal of Physiology-Endocrinology and Metabolism 279(3):E501-507. https://doi.org/10.1152/ajpendo.2000.279.3.E501
- Kwon, D.R., et al. (2015). Effect of Intra-articular Injection of AOD9604 with or without Hyaluronic Acid in Rabbit Osteoarthritis Model. Annals of Clinical and Laboratory Science 45(4):426-432. https://pubmed.ncbi.nlm.nih.gov/26275694/
- Cox, H.D., et al. (2015). Detection and in vitro metabolism of AOD9604. Drug Testing and Analysis 7(1):31-38. https://doi.org/10.1002/dta.1715
- Stier, H., et al. (2013). Safety and Tolerability of the Hexadecapeptide AOD9604 in Humans. Journal of Endocrinology and Metabolism 3(1-2):7-15. https://jofem.org/index.php/jofem/article/view/157
- Moré, M.I., et al. (2014). Safety and Metabolism of AOD9604, a Novel Nutraceutical Ingredient for Improved Metabolic Health. Journal of Endocrinology and Metabolism 4(3):64-77. https://jofem.org/index.php/jofem/article/view/213
- Habibullah, M.M., et al. (2022). Human Growth Hormone Fragment 176-191 Peptide Enhances the Toxicity of Doxorubicin-Loaded Chitosan Nanoparticles Against MCF-7 Breast Cancer Cells. Drug Design, Development and Therapy 16:1963-1974. https://doi.org/10.2147/DDDT.S372450
- Orlovius, A.K., et al. (2013). AOD-9604 does not influence the WADA hGH isoform immunoassay. Drug Testing and Analysis 5(11-12):810-814. https://doi.org/10.1002/dta.1557
- Kim, S.W., et al. (2005). Growth hormone treatment and visceral adiposity in adults. Growth Hormone & IGF Research 15(2):S49-S57. https://doi.org/10.1016/j.ghir.2005.02.006
- World Anti-Doping Agency. (2024). The Prohibited List 2025. https://www.wada-ama.org/sites/default/files/2024-09/2025list_en_final_clean_12_september_2024.pdf
- USADA. (2024). World Anti-Doping Agency (WADA) Prohibited List. https://www.usada.org/athletes/substances/prohibited-list/
- Bengtsson, B.A., et al. (1993). Treatment of adults with growth hormone (GH) deficiency with recombinant human GH. Journal of Clinical Endocrinology & Metabolism 76(2):309-317. https://doi.org/10.1210/jcem.76.2.8432773
Last reviewed: October 2025
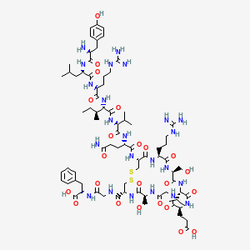
| CAS Number | 221231-10-3 |
|---|---|
| Molecular Formula | C78H123N23O23S2 |
| Molecular Weight | 1815.1 g/Mol |
| Sequence | H-Tyr-Leu-Arg-Ile-Val-Gln-Cys(1)-Arg-Ser-Val-Glu-Gly-Ser-Cys(1)-Gly-Phe-OH |
Cosmic Peptides
Certificate of Analysis
Unable to load certificate. Download COA





