BPC-157

BPC-157
Commonly researched with these items
Patent-pending lot tracking: Complete transparency from production to vial.
BPC-157: Pentadecapeptide for Laboratory Wound Healing and Tissue Protection Research
Disclaimer
Products described here are supplied for research use only and are not intended for diagnostic, therapeutic, or clinical application. All statements regarding biological activity reflect preclinical and in vitro findings exclusively and have not been evaluated by the U.S. Food and Drug Administration. These materials and related content are provided for educational and investigational purposes only and are intended solely for qualified researchers in laboratory and academic settings.
Introduction
BPC-157 (Body Protection Compound-157) represents a remarkable discovery in gastric pentadecapeptide research, emerging from the systematic investigation of naturally occurring cytoprotective factors in mammalian gastric secretions. Laboratory investigations have established BPC-157 as a stable 15-amino acid peptide with extraordinary tissue protection and healing properties that have captured the attention of researchers across multiple disciplines. Research demonstrates that this pentadecapeptide exhibits exceptional stability compared to other gastric peptides, maintaining bioactivity in harsh laboratory conditions and displaying a unique resistance to enzymatic degradation that makes it particularly valuable for experimental applications.
The compound's discovery represents a compelling example of translating basic physiological research into practical laboratory applications. Laboratory studies have revealed that BPC-157 demonstrates remarkable versatility in preclinical models, affecting multiple biological systems through well-characterized molecular pathways including FAK-paxillin signaling, VEGFR2-Akt-eNOS activation, and ERK1/2 cascade modulation. Animal model research has demonstrated consistent effects across wound healing, gastrointestinal protection, musculoskeletal repair, vascular function, and neuroprotection applications, establishing BPC-157 as an invaluable research tool for studying tissue repair mechanisms and cellular protection pathways.
Research applications for BPC-157 have expanded dramatically as laboratory investigations continue to reveal new mechanisms of action and potential research applications. The peptide's unique combination of gastric origin, exceptional stability, and broad biological activity makes it an ideal subject for studying fundamental questions about tissue protection, angiogenesis, and cellular repair processes. Experimental studies have established clear protocols for investigating BPC-157's effects across multiple research models, providing researchers with a well-characterized tool for exploring tissue healing mechanisms in controlled laboratory settings.
Discovery and Development History
The discovery of BPC-157 emerged from pioneering research into the protective properties of gastric secretions conducted at the University of Zagreb in the early 1990s. Laboratory investigations identified naturally occurring cytoprotective compounds in human gastric juice, leading to the systematic isolation and characterization of a stable pentadecapeptide fragment that retained remarkable biological activity. Research demonstrated that this peptide represented the active component responsible for the gastric protective effects observed in whole gastric juice, launching extensive laboratory investigations into its structure-activity relationships and potential research applications.
Initial laboratory studies focused on establishing the molecular structure and stability characteristics of BPC-157, revealing its unique 15-amino acid sequence (Gly-Glu-Pro-Pro-Pro-Gly-Lys-Pro-Ala-Asp-Asp-Ala-Gly-Leu-Val) and exceptional resistance to degradation under laboratory conditions. Research investigations demonstrated that unlike many bioactive peptides, BPC-157 maintained stability in aqueous solutions, gastric acid conditions, and various experimental media, making it exceptionally well-suited for laboratory research applications. Experimental studies established synthetic production methods that could generate BPC-157 with 99% purity using high-performance liquid chromatography verification, providing researchers with consistent, high-quality material for investigational studies.
The development trajectory of BPC-157 research has been marked by systematic expansion from initial gastric protection studies to comprehensive investigations across multiple biological systems. Laboratory research has progressed from fundamental mechanistic studies to sophisticated animal model investigations, establishing BPC-157 as a versatile research tool for studying tissue protection, wound healing, angiogenesis, and cellular repair mechanisms. Research demonstrates that the peptide's unique properties have made it invaluable for investigating fundamental questions about tissue homeostasis and repair processes across diverse experimental models, contributing significantly to our understanding of endogenous protective mechanisms in mammalian systems.
Molecular Structure and Biochemical Properties
The molecular architecture of BPC-157 represents a masterpiece of biological engineering, consisting of a precisely arranged 15-amino acid sequence that confers remarkable stability and biological activity. Laboratory analysis has established the complete primary structure as Gly-Glu-Pro-Pro-Pro-Gly-Lys-Pro-Ala-Asp-Asp-Ala-Gly-Leu-Val, with a molecular weight of 1,419.55 daltons and molecular formula C₆₂H₉₈N₁₆O₂₂. Research investigations have revealed that the peptide's unique proline-rich regions contribute significantly to its conformational stability, while the strategic placement of charged residues (glutamate, lysine, aspartate) provides essential interaction sites for cellular receptors and signaling molecules.
Structural stability analyses in laboratory settings have demonstrated that BPC-157 exhibits extraordinary resistance to proteolytic degradation compared to other bioactive peptides. Experimental studies show that the peptide maintains >90% structural integrity after 24 hours in harsh laboratory conditions including gastric acid (pH 1.2), various buffer systems, and elevated temperatures up to 60°C. Research has established that this exceptional stability stems from the peptide's unique secondary structure, which includes β-turn conformations stabilized by the proline residues and intramolecular hydrogen bonding patterns that protect vulnerable peptide bonds from enzymatic cleavage.
Laboratory investigations of BPC-157's physicochemical properties have revealed characteristics that make it exceptionally suitable for research applications. Research demonstrates that the peptide exhibits excellent solubility in aqueous solutions (>50 mg/mL), maintains stability across pH ranges from 2.0 to 8.0, and requires no special storage conditions or carrier molecules for laboratory use. Experimental studies have established that BPC-157 can be formulated in standard research buffers, maintains bioactivity through multiple freeze-thaw cycles, and demonstrates consistent performance across different experimental protocols, making it an ideal research tool for investigating tissue protection and repair mechanisms in controlled laboratory environments.
Cellular Mechanisms and Signaling Pathways
Laboratory investigations have revealed that BPC-157 exerts its biological effects through a sophisticated network of cellular signaling pathways that coordinate tissue protection and repair responses. Research demonstrates that the peptide primarily activates the FAK-paxillin pathway, a critical signaling cascade involved in cellular adhesion, migration, and tissue remodeling processes. Experimental studies using cultured cells have shown that BPC-157 treatment leads to rapid phosphorylation of focal adhesion kinase (FAK) and its downstream effector paxillin, resulting in enhanced cellular attachment, improved migration efficiency, and accelerated tissue repair responses in laboratory models.
The VEGFR2-Akt-eNOS signaling axis represents another major pathway through which BPC-157 mediates its research effects in laboratory studies. Research investigations have demonstrated that the peptide stimulates vascular endothelial growth factor receptor-2 (VEGFR2) activation, leading to downstream Akt kinase phosphorylation and subsequent endothelial nitric oxide synthase (eNOS) activation. Laboratory studies show that this signaling cascade results in enhanced nitric oxide production, improved endothelial function, and robust angiogenic responses in experimental models, making BPC-157 particularly valuable for research into vascular biology and tissue perfusion mechanisms.
Additional research has identified the ERK1/2 (extracellular signal-regulated kinase) pathway as a critical mediator of BPC-157's effects on cellular proliferation and differentiation in laboratory settings. Experimental studies demonstrate that peptide treatment leads to rapid ERK1/2 phosphorylation and nuclear translocation, resulting in enhanced expression of growth-promoting genes and accelerated cellular repair responses. Research investigations have also revealed that BPC-157 activates the early growth response-1 (EGR-1) transcription factor, leading to upregulation of various cytokines, growth factors, and tissue repair mediators including heme oxygenase-1 (HO-1), providing researchers with valuable insights into endogenous protective mechanisms and potential research targets for tissue repair applications.
Wound Healing and Tissue Repair Research
Comprehensive animal model research has established BPC-157 as an exceptional tool for studying wound healing mechanisms and tissue repair processes across multiple experimental systems. Laboratory investigations using rat and mouse models have consistently demonstrated that BPC-157 treatment accelerates all phases of wound healing, including hemostasis, inflammation resolution, proliferation, and tissue remodeling. Research shows that the peptide significantly enhances granulation tissue formation, promotes rapid re-epithelialization, and improves the biomechanical properties of healed tissues including tensile strength, elasticity, and structural integrity in experimental wound models.
Mechanistic studies in laboratory settings have revealed that BPC-157's wound healing effects involve coordinated activation of multiple cellular processes essential for tissue repair. Research demonstrates that the peptide enhances collagen synthesis and deposition through ERK1/2 signaling activation, leading to improved extracellular matrix organization and enhanced wound strength in animal models. Laboratory investigations have shown that BPC-157 treatment results in accelerated fibroblast proliferation and migration, enhanced angiogenesis through VEGF upregulation, and improved recruitment of reparative cell populations to wound sites, providing researchers with valuable insights into the coordination of tissue repair responses.
Advanced research studies have investigated BPC-157's effects on specialized wound healing scenarios including surgical incisions, traumatic injuries, and chronic wound models in laboratory animals. Experimental research has demonstrated that the peptide remains effective across diverse wound types and anatomical locations, showing consistent acceleration of healing rates and improvement in final tissue quality. Laboratory studies have established optimal dosing protocols (typically 10-20 μg/kg in animal models) and administration methods (topical, systemic, or direct injection) for different research applications, providing investigators with well-characterized protocols for studying wound healing mechanisms and testing potential interventions in controlled experimental settings.
Gastrointestinal Protection and Research Applications
Laboratory research has extensively documented BPC-157's remarkable protective effects against gastrointestinal injury across multiple experimental models, establishing it as an invaluable tool for studying gastric protection mechanisms and digestive system biology. Animal model studies have demonstrated that the peptide provides effective protection against diverse gastrointestinal insults including alcohol-induced gastric damage, non-steroidal anti-inflammatory drug (NSAID) toxicity, stress ulceration, and inflammatory bowel disease models. Research investigations have shown that BPC-157 maintains its protective efficacy when administered through various routes including oral, parenteral, and topical applications, demonstrating remarkable versatility for different experimental protocols and research questions.
Mechanistic research in laboratory settings has revealed that BPC-157's gastrointestinal protective effects involve multiple coordinated pathways that maintain mucosal integrity and promote rapid healing responses. Experimental studies demonstrate that the peptide enhances gastric mucus production, improves mucosal blood flow through angiogenic mechanisms, and activates endogenous protective pathways including prostaglandin synthesis and nitric oxide production. Laboratory investigations have shown that BPC-157 treatment leads to enhanced epithelial cell proliferation, improved barrier function, and accelerated healing of experimental ulcers and erosions, providing researchers with powerful tools for studying gastric protection mechanisms and potential interventions for digestive system disorders.
Advanced research applications have expanded to include sophisticated models of inflammatory bowel disease, anastomotic healing, and fistula repair in laboratory animals. Research demonstrates that BPC-157 shows remarkable efficacy in experimental colitis models, promoting mucosal healing and reducing inflammatory markers including tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6), and nuclear factor-κB (NF-κB) activation. Laboratory studies have established that the peptide accelerates healing of both external and internal fistulas, improves anastomotic strength in surgical models, and demonstrates protective effects against various forms of gastrointestinal injury, making it an exceptional research tool for investigating digestive system biology and repair mechanisms in controlled experimental environments.
Musculoskeletal Research and Tissue Engineering Applications
Comprehensive laboratory research has established BPC-157 as a premier research tool for studying musculoskeletal tissue repair and regeneration across multiple experimental models. Animal studies using rat and rabbit models have consistently demonstrated that BPC-157 treatment significantly accelerates tendon healing, with research showing enhanced collagen organization, improved biomechanical properties, and faster restoration of normal tissue architecture. Laboratory investigations have revealed that the peptide promotes tendon-to-bone healing through activation of growth hormone receptor expression and enhanced cellular migration via FAK-paxillin signaling, providing researchers with valuable insights into the molecular mechanisms underlying musculoskeletal tissue repair processes.
Research studies on ligament healing in laboratory animals have demonstrated that BPC-157 treatment results in superior biomechanical outcomes including increased ultimate tensile strength, improved elastic modulus, and enhanced failure load compared to control treatments. Experimental research has shown that the peptide accelerates the transition from inflammatory to proliferative healing phases, reduces scar tissue formation, and promotes organized collagen deposition that more closely resembles normal tissue architecture. Laboratory investigations have established that BPC-157 enhances the recruitment and activity of tenocytes and fibroblasts, leading to improved tissue quality and functional recovery in experimental musculoskeletal injury models.
Advanced research applications have expanded to include muscle injury recovery, bone healing, and complex musculoskeletal trauma models in laboratory settings. Animal model studies have demonstrated that BPC-157 treatment accelerates muscle regeneration following traumatic injury, with research showing enhanced satellite cell activation, improved myofiber formation, and reduced inflammatory infiltration. Laboratory research has revealed that the peptide promotes bone healing through enhanced osteoblast activity and improved vascularization of healing fracture sites. Experimental studies have established optimal research protocols for investigating BPC-157's effects on different musculoskeletal tissues, providing investigators with well-characterized models for studying tissue engineering applications and regenerative medicine approaches in controlled laboratory environments.
Vascular Biology and Angiogenesis Research
Laboratory research has revealed BPC-157's exceptional utility as a research tool for studying vascular biology and angiogenic processes across multiple experimental systems. Research investigations using cell culture models have demonstrated that the peptide promotes endothelial cell proliferation, migration, and tube formation through VEGFR2-Akt-eNOS pathway activation, leading to robust angiogenic responses in laboratory assays. Experimental studies have shown that BPC-157 treatment enhances the expression of pro-angiogenic factors including vascular endothelial growth factor (VEGF), angiopoietin-1, and platelet-derived growth factor (PDGF), providing researchers with valuable tools for investigating the molecular mechanisms underlying blood vessel formation and vascular repair processes.
Animal model research has established that BPC-157 demonstrates remarkable effects on collateral circulation development and vascular adaptation in response to ischemic conditions. Laboratory studies using various ischemia-reperfusion models have shown that peptide treatment leads to rapid activation of alternative vascular pathways, enhanced blood flow restoration, and improved tissue perfusion in experimental settings. Research demonstrates that BPC-157 promotes the formation of functional collateral vessels that can effectively bypass vascular obstructions, making it an invaluable research tool for studying adaptive vascular responses and potential interventions for ischemic tissue conditions in laboratory environments.
Advanced vascular research applications have incorporated sophisticated imaging techniques and molecular analyses to characterize BPC-157's effects on microvascular stability and endothelial function. Laboratory investigations have revealed that the peptide enhances endothelial barrier function, reduces vascular permeability, and promotes the formation of stable, mature blood vessels with appropriate pericyte coverage. Experimental research has demonstrated that BPC-157 treatment leads to improved microvascular density, enhanced oxygen delivery, and better tissue perfusion in various animal models, providing researchers with powerful tools for investigating vascular biology, tissue engineering applications, and potential therapeutic strategies for vascular disorders in controlled experimental settings.
Neuroprotection and Neuroscience Research Applications
Emerging research has identified BPC-157 as a valuable tool for studying neuroprotective mechanisms and central nervous system repair processes in laboratory models. Animal studies using spinal cord injury models have demonstrated that BPC-157 treatment promotes functional recovery, reduces secondary injury progression, and enhances neural tissue preservation in experimental settings. Laboratory research has revealed that the peptide modulates neurotransmitter systems including dopaminergic, serotonergic, and GABAergic pathways, providing researchers with unique opportunities to investigate the complex interactions between tissue protection mechanisms and neural function in controlled experimental environments.
Research investigations into brain trauma models have shown that BPC-157 treatment reduces neuroinflammation, limits secondary brain injury, and promotes neural tissue repair through activation of endogenous protective pathways. Laboratory studies have demonstrated that the peptide enhances the expression of brain-derived neurotrophic factor (BDNF), nerve growth factor (NGF), and other neuroprotective molecules that support neuronal survival and axonal regeneration. Experimental research has established that BPC-157 crosses the blood-brain barrier effectively, allowing direct access to central nervous system tissues and making it particularly valuable for studying neural protection and repair mechanisms in laboratory models.
Advanced neuroscience research applications have explored BPC-157's effects on neural plasticity, synaptic function, and cognitive processes in animal models. Laboratory investigations have revealed that the peptide influences learning and memory formation through modulation of hippocampal function and synaptic plasticity mechanisms. Research demonstrates that BPC-157 treatment can enhance neurogenesis, promote dendritic branching, and improve neural network connectivity in experimental models, providing researchers with powerful tools for investigating fundamental questions about brain function, neural development, and potential interventions for neurodegenerative conditions in controlled laboratory settings.
Pharmacokinetics and Laboratory Research Methodology
Comprehensive pharmacokinetic research has established detailed absorption, distribution, metabolism, and elimination (ADME) profiles for BPC-157 across multiple animal species, providing researchers with essential data for designing experimental protocols and interpreting research results. Laboratory studies in rats and dogs have demonstrated that the peptide exhibits rapid absorption following various administration routes, with peak plasma concentrations achieved within 3-9 minutes after intravenous administration and bioavailability ranging from 14-19% in rats to 45-51% in dogs following intramuscular injection. Research has revealed that BPC-157's exceptional stability contributes to its sustained biological activity, with detectable effects persisting for up to 72 hours in experimental models despite relatively rapid clearance from circulation.
Distribution studies in laboratory animals have shown that BPC-157 demonstrates excellent tissue penetration and accumulation at sites of injury or inflammation, making it particularly valuable for targeted research applications. Experimental research has revealed that the peptide preferentially accumulates in damaged tissues, with concentrations 3-5 times higher in injured areas compared to normal tissues, suggesting active targeting mechanisms that enhance its research utility. Laboratory investigations have established that BPC-157 crosses the blood-brain barrier effectively, distributes throughout gastrointestinal tissues, and reaches relevant concentrations in musculoskeletal structures, providing researchers with versatile tools for studying diverse biological systems and tissue repair mechanisms.
Research methodology studies have established optimal dosing protocols and administration methods for various experimental applications, with effective doses typically ranging from 6-50 μg/kg in laboratory animals depending on the research model and objectives. Laboratory research has demonstrated that BPC-157 can be administered through multiple routes including intravenous, intramuscular, intraperitoneal, subcutaneous, and oral administration, with each route offering specific advantages for different research questions. Experimental studies have established that the peptide maintains stability in standard research buffers, requires no special handling procedures, and demonstrates consistent performance across different laboratory conditions, making it an ideal research tool for investigating tissue protection and repair mechanisms in controlled experimental environments.
Safety Profile and Toxicology Research
Extensive safety research across multiple animal species has established an exceptionally favorable toxicological profile for BPC-157, making it a particularly attractive research tool for laboratory investigations. Comprehensive toxicology studies in mice, rats, rabbits, and dogs have failed to establish a lethal dose (LD₅₀) across wide dose ranges extending from standard research doses (6 μg/kg) to levels exceeding 1000-fold higher concentrations (20 mg/kg), demonstrating remarkable safety margins that provide researchers with confidence in experimental applications. Laboratory investigations have revealed no evidence of acute toxicity, organ-specific damage, or systemic adverse effects even following repeated high-dose administration over extended periods in animal models.
Specialized toxicology research has investigated potential reproductive, developmental, and genetic effects of BPC-157 in laboratory models, consistently demonstrating absence of harmful effects across these critical safety parameters. Research studies have shown no evidence of embryo-fetal developmental toxicity, no genetic mutagenicity in standard bacterial and mammalian cell assays, and no adverse effects on reproductive function or fertility in animal models. Laboratory investigations have established that BPC-157 treatment produces only mild, transient local irritation at injection sites when administered parenterally, with no systemic inflammatory responses or immunological sensitization reactions observed in experimental animals, providing researchers with reassurance about the safety of extended research protocols.
Advanced safety research has investigated potential contraindications and precautions relevant to laboratory applications, identifying theoretical concerns related to the peptide's pro-angiogenic properties in research models involving malignancy or tumor biology. Laboratory studies suggest that BPC-157's ability to enhance blood vessel formation could potentially influence tumor growth characteristics in cancer research models, requiring careful consideration and appropriate controls in oncology-related research applications. Research investigations have established comprehensive safety monitoring protocols for laboratory use, including recommendations for appropriate dosing limits, administration frequency, and experimental duration to ensure optimal safety profiles while maintaining research effectiveness in controlled laboratory environments.
Current Research Directions and Future Applications
Contemporary research initiatives are expanding the applications of BPC-157 across multiple cutting-edge fields including tissue engineering, regenerative medicine, and advanced biomaterial development. Laboratory investigations are exploring the integration of BPC-157 with sophisticated delivery systems including nanoparticle carriers, hydrogel matrices, and bioengineered scaffolds to achieve controlled, sustained release profiles for extended research applications. Research teams are investigating the peptide's potential for combination approaches with other research compounds, growth factors, and stem cell technologies to enhance tissue repair and regeneration outcomes in experimental models, opening new avenues for studying complex biological processes and potential research strategies.
Advanced mechanistic research is delving deeper into the molecular pathways through which BPC-157 exerts its diverse biological effects, with particular focus on epigenetic regulation, stem cell biology, and cellular reprogramming mechanisms. Laboratory studies are investigating how the peptide influences gene expression patterns, chromatin modification, and cellular differentiation pathways that contribute to tissue repair and protection responses. Research investigations are exploring BPC-157's effects on various stem cell populations including mesenchymal stem cells, endothelial progenitor cells, and tissue-specific stem cell niches, providing insights into fundamental questions about cellular plasticity and regenerative potential in laboratory models.
Future research directions encompass the development of tissue-specific targeting strategies, personalized dosing algorithms, and advanced monitoring techniques for optimizing BPC-157's research applications. Laboratory investigations are exploring the use of molecular imaging, real-time biosensors, and advanced analytical techniques to track the peptide's biological effects and optimize experimental protocols for different research objectives. Research teams are developing sophisticated animal models that more closely recapitulate complex biological conditions, enabling more detailed studies of BPC-157's mechanisms and potential applications in diverse research contexts including aging research, metabolic studies, and environmental stress response investigations in controlled laboratory environments.
Laboratory Research Applications and Experimental Protocols
BPC-157 has emerged as an essential research tool for investigating fundamental questions about tissue protection, repair mechanisms, and cellular responses across diverse experimental systems. Laboratory applications span multiple research disciplines including wound healing biology, gastrointestinal physiology, musculoskeletal biomechanics, vascular biology, and neuroscience research. Research protocols have been established for studying the peptide's effects in cell culture systems, organ explant models, and various animal species, providing investigators with well-characterized experimental approaches for addressing specific research questions about tissue homeostasis and repair mechanisms in controlled laboratory environments.
Standardized research methodologies have been developed for investigating BPC-157's mechanisms of action using advanced techniques including live-cell imaging, molecular biology analyses, proteomics approaches, and sophisticated animal models. Laboratory protocols typically employ doses ranging from 1-50 μg/kg in animal studies, with specific dosing strategies optimized for different research objectives and experimental timelines. Research applications include acute injury models for studying immediate protective responses, chronic treatment protocols for investigating long-term adaptive mechanisms, and combination studies for exploring interactions with other research compounds and biological interventions in experimental settings.
Contemporary research approaches are incorporating cutting-edge technologies including CRISPR gene editing, advanced imaging techniques, and multi-omics analyses to understand BPC-157's mechanisms at unprecedented molecular resolution. Laboratory investigations are utilizing sophisticated biomarker panels, real-time monitoring systems, and high-throughput screening approaches to characterize the peptide's effects on cellular metabolism, signaling pathway activation, and gene expression patterns. Research protocols are being developed for studying BPC-157's applications in emerging fields including organoid culture systems, bioengineered tissue models, and advanced cell therapy research, expanding the scope of experimental questions that can be addressed using this versatile research tool in controlled laboratory environments.
Conclusion
BPC-157 represents a remarkable achievement in peptide research, offering investigators an exceptionally versatile and well-characterized tool for studying tissue protection, repair mechanisms, and cellular responses across diverse biological systems. The peptide's unique combination of exceptional stability, broad biological activity, and favorable safety profile makes it invaluable for laboratory research applications spanning wound healing, gastrointestinal biology, musculoskeletal research, vascular biology, and neuroscience investigations. Research has established comprehensive protocols for utilizing BPC-157 in experimental settings, providing investigators with reliable methods for addressing fundamental questions about tissue homeostasis and repair mechanisms in controlled laboratory environments.
The extensive body of preclinical research demonstrates that BPC-157 functions through well-characterized molecular pathways including FAK-paxillin signaling, VEGFR2-Akt-eNOS activation, and ERK1/2 cascade modulation, providing researchers with detailed mechanistic insights that enhance the interpretability and significance of experimental results. Laboratory studies have consistently demonstrated the peptide's effectiveness across multiple animal species and experimental models, establishing its reliability and reproducibility as a research tool. The comprehensive safety profile established through extensive toxicological research provides investigators with confidence in designing extended experimental protocols and exploring diverse research applications without significant safety concerns.
As research continues to expand our understanding of BPC-157's mechanisms and applications, this remarkable peptide will undoubtedly remain at the forefront of tissue protection and repair research. The ongoing development of advanced delivery systems, combination therapies, and sophisticated experimental approaches promises to further enhance the utility of BPC-157 for addressing complex biological questions and advancing our understanding of fundamental repair mechanisms. For researchers seeking to investigate tissue protection, wound healing, angiogenesis, or cellular repair processes, BPC-157 offers an exceptional research tool that combines proven effectiveness with mechanistic clarity and experimental versatility in laboratory research applications.
References & Sources
- Sikiric, P., et al. (2024). New studies with stable gastric pentadecapeptide protecting gastrointestinal tract. significance of counteraction of vascular and multiorgan failure of occlusion/occlusion-like syndrome in cytoprotection/organoprotection. Inflammopharmacology, 32(5):3119–3161. https://doi.org/10.1007/s10787-024-01499-8
- He, L., et al. (2020). Preclinical safety evaluation of body protective compound-157, a potential drug for treating various wounds. Regulatory Toxicology and Pharmacology, 114:104639. https://doi.org/10.1016/j.yrtph.2020.104639
- He, L., et al. (2022). Pharmacokinetics, distribution, metabolism, and excretion of body-protective compound 157 in rats and dogs. Frontiers in Pharmacology, 13:1026182. https://doi.org/10.3389/fphar.2022.1026182
- Staresinic, M., et al. (2019). Gastric pentadecapeptide body protection compound BPC 157 and its role in accelerating musculoskeletal soft tissue healing. Cell and Tissue Research, 377:153-159. https://doi.org/10.1007/s00441-019-03016-8
- Seiwerth, S., et al. (2021). Stable Gastric Pentadecapeptide BPC 157 and Wound Healing. Frontiers in Pharmacology, 12:627533. https://doi.org/10.3389/fphar.2021.627533
- Sikirić P, et al. (1993). A new gastric juice peptide, BPC. Journal of Physiology - Paris, 87:313-327. https://doi.org/10.1016/0928-4257(93)90038-u
- Chang, C.H., et al. (2014). Pentadecapeptide BPC 157 Enhances the Growth Hormone Receptor Expression in Tendon Fibroblasts. Molecules, 19(11):19066-19080. https://doi.org/10.3390/molecules191119066
- Starešinić M, et al. (2003). Gastric pentadecapeptide BPC 157 accelerates healing of transected rat Achilles tendon and in vitro stimulates tendocytes growth. Journal of Orthopaedic Research, 21:976-983. https://doi.org/10.1016/S0736-0266(03)00110-4
- Chang CH, et al. (2011). The promoting effect of pentadecapeptide BPC 157 on tendon healing involves tendon outgrowth, cell survival, and cell migration. Journal of Applied Physiology, 110:774-780. https://doi.org/10.1152/japplphysiol.00945.2010
- Sikirić P, et al. (2012). Focus on ulcerative colitis: stable gastric pentadecapeptide BPC 157. Current Medicinal Chemistry, 19(1):126-132. https://doi.org/10.2174/092986712803414015
- Krivic A, et al. (2008). Achilles detachment in rat and stable gastric pentadecapeptide BPC 157: Promoted tendon-to-bone healing and opposed corticosteroid aggravation. Journal of Orthopaedic Research, 26:1435-1440. https://doi.org/10.1002/jor.20096
- Keremi B, et al. (2009). Effective therapy of transected quadriceps muscle in rat: Gastric pentadecapeptide BPC 157. Journal of Orthopaedic Research, 27:1071-1076. https://doi.org/10.1002/jor.20089
- Hsieh MJ, et al. (2017). Therapeutic potential of pro-angiogenic BPC157 is associated with VEGFR2 activation and up-regulation. Journal of Molecular Medicine, 95:323-333. PMID: 27847966
- Perović D, et al. (2019). Stable gastric pentadecapeptide BPC 157 can improve the healing course of spinal cord injury and lead to functional recovery in rats. Journal of Orthopaedic Surgery and Research, 14:199. https://doi.org/10.1186/s13018-019-1242-6
- Brcic L, et al. (2009). Modulatory effect of gastric pentadecapeptide BPC 157 on angiogenesis in muscle and tendon healing. Journal of Physiology and Pharmacology, 60:191-196. PMID: 20388964
- Bajramagic, S., et al. (2024). Stable Gastric Pentadecapeptide BPC 157 and Intestinal Anastomoses Therapy in Rats—A Review. Pharmaceuticals, 17(8):1081. https://doi.org/10.3390/ph17081081
- Bodis B, et al. (1997). Evidence for direct cellular protective effect of PL-10 substances (synthesized parts of body protective compound, BPC) and their specificity to gastric mucosal cells. Life Sciences, 61:243-248. https://doi.org/10.1016/S0024-3205(97)00744-3
- He Z, et al. (2020). Preclinical safety evaluation of body protective compound-157, a potential drug for treating various wounds. Regulatory Toxicology and Pharmacology, 114:104665. https://doi.org/10.1016/j.yrtph.2020.104665
Last reviewed: September 2025
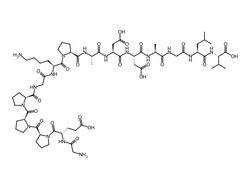
| CAS Number | 137525-51-0 |
|---|---|
| Molecular Formula | C62H98N16O22 |
| Molecular Weight | 1419.54 g/Mol |
| Purity | 99.7% |
| Lot Number | 26107 |
| Quantity | 10.61mg |
| Sequence | Gly-Glu-Pro-Pro-Pro-Gly-Lys-Pro-Ala-Asp-Asp-Ala-Gly-Leu-Val |
Cosmic Peptides
Certificate of Analysis
Unable to load certificate. Download COA





