DSIP

DSIP
Commonly researched with these items
Patent-pending lot tracking: Complete transparency from production to vial.
DSIP (Delta Sleep-Inducing Peptide)
Disclaimer
Products described here are supplied for research use only and are not intended for diagnostic, therapeutic, or clinical application. All statements regarding biological activity reflect preclinical and in vitro findings exclusively and have not been evaluated by the U.S. Food and Drug Administration. These materials and related content are provided for educational and investigational purposes only and are intended solely for qualified researchers in laboratory and academic settings.
Introduction
Delta Sleep-Inducing Peptide (DSIP) represents one of the most enigmatic and multifaceted neuropeptides in modern neuroscience research, having captivated investigators for nearly five decades since its initial discovery. First isolated in 1974-1977 by the pioneering research team of Ernst Schoenenberger and Marcel Monnier at the University of Basel, DSIP emerged from groundbreaking experiments investigating the biochemical basis of sleep regulation. The peptide was originally extracted from the venous blood of rabbits subjected to electrical stimulation of the thalamic sleep-inducing centers, representing the first successful isolation of an endogenous sleep-promoting factor. This discovery marked a watershed moment in sleep research, providing the first molecular evidence that sleep could be induced by specific biochemical signals rather than merely representing the absence of wakefulness.
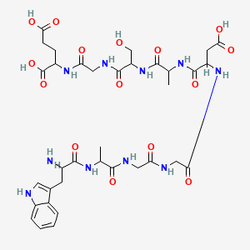
DSIP consists of nine amino acids arranged in the sequence Tryptophan-Alanine-Glycine-Glycine-Aspartic acid-Alanine-Serine-Glycine-Glutamic acid (Trp-Ala-Gly-Gly-Asp-Ala-Ser-Gly-Glu), with a molecular weight of 849 daltons. What distinguishes DSIP from virtually all other bioactive peptides is the remarkable paradox that surrounds its existence: despite nearly fifty years of intensive research, no specific receptor, precursor protein, or encoding gene has been definitively identified for this biologically active nonapeptide. This scientific enigma has led researchers to describe DSIP as "a still unresolved riddle" and "the most mysterious peptide in neuroscience," yet its profound biological effects across multiple physiological systems remain undeniably documented in hundreds of peer-reviewed studies.
The biological significance of DSIP extends far beyond its original identification as a sleep-inducing factor. Research has revealed that this remarkable peptide exhibits an unprecedented breadth of physiological activities, including modulation of sleep architecture, stress response regulation, pain perception, temperature control, immune function, and addiction recovery processes. Unlike most neuropeptides that exhibit highly specific receptor-mediated actions, DSIP appears to function through multiple, as-yet-unidentified mechanisms that allow it to influence diverse physiological systems. This unique profile has made DSIP an invaluable research tool for investigating the fundamental relationships between sleep, stress, and overall physiological homeostasis, while simultaneously presenting one of the most compelling unsolved mysteries in modern biochemistry.

Figure: Molecular structure of DSIP showing the nonapeptide sequence (Trp-Ala-Gly-Gly-Asp-Ala-Ser-Gly-Glu). The N-terminal tryptophan residue and the central glycine-rich region are critical for biological activity, though the exact mechanism of action remains one of neuroscience's greatest unsolved puzzles.
The DSIP Paradox: Function Without Known Receptor
The central mystery surrounding DSIP lies in the unprecedented paradox of demonstrable biological activity without identifiable receptor mechanisms—a situation virtually unique in modern peptide pharmacology. Despite extensive research efforts spanning five decades, employing increasingly sophisticated molecular biology techniques, no specific DSIP receptor has been characterized, cloned, or even convincingly identified. This absence of receptor identification stands in stark contrast to the well-documented biological effects of the peptide, creating what researchers have termed "the DSIP paradox." Traditional approaches to peptide research rely on receptor identification as the foundation for understanding mechanism of action, yet DSIP continues to exert profound physiological effects through pathways that remain fundamentally mysterious.
The search for DSIP's mechanism of action has yielded tantalizing clues without definitive answers. Research has demonstrated that DSIP can modulate intracellular cyclic adenosine monophosphate (cAMP) levels in various cell types, suggesting interaction with G-protein-coupled signaling systems, yet no specific G-protein-coupled receptor for DSIP has been identified. Similarly, studies have shown that DSIP influences ion channel activity, particularly calcium and potassium channels, in neuronal preparations, but these effects appear to be indirect rather than resulting from direct channel binding. Some investigators have proposed that DSIP may function through novel, non-receptor-mediated mechanisms, potentially involving direct membrane interactions or modulation of membrane fluidity properties. However, these hypotheses remain largely speculative and lack the experimental validation that characterizes established peptide mechanisms.
The absence of an identified DSIP gene or precursor protein compounds the mystery surrounding this peptide's biological origin and regulation. Unlike virtually all other bioactive peptides, which arise from well-characterized precursor proteins through specific enzymatic cleavage processes, DSIP's biosynthetic pathway remains completely unknown. Extensive genomic searches using modern bioinformatics approaches have failed to identify any gene encoding DSIP or a related precursor, leading some researchers to speculate that the peptide might arise through non-ribosomal synthetic pathways or represent a breakdown product of larger proteins. This fundamental gap in our understanding has profound implications for DSIP research, as the inability to study gene expression, regulation, or knockout models severely limits traditional molecular approaches to understanding the peptide's physiological role. The persistence of these mysteries has made DSIP research a unique frontier in neuroscience, where empirical observation of biological effects must proceed without the theoretical framework that guides most modern peptide research.
Sleep Architecture and Circadian Rhythm Modulation
DSIP's most extensively documented and originally discovered biological activity involves its profound effects on sleep architecture and the regulation of circadian rhythms. Sleep research studies have consistently demonstrated that DSIP administration in animal models produces specific alterations in sleep patterns that distinguish it from conventional sedatives or hypnotics. Rather than simply inducing unconsciousness, DSIP appears to enhance the natural sleep process by specifically promoting delta-wave sleep (stages 3 and 4 of non-REM sleep), the deepest and most restorative phases of the sleep cycle. Electroencephalographic (EEG) studies in experimental animals show that DSIP increases delta-wave activity by approximately 35% compared to baseline measurements, while simultaneously improving sleep efficiency and reducing sleep fragmentation.
The sleep-promoting effects of DSIP exhibit several unique characteristics that differentiate it from other sleep-inducing compounds. Unlike benzodiazepines or other GABA-ergic sleep aids, DSIP does not suppress REM sleep or alter the natural progression through sleep stages. Instead, research demonstrates that DSIP preserves normal sleep architecture while enhancing the depth and quality of slow-wave sleep phases in animal models. Sleep latency studies show that DSIP reduces the time required to fall asleep by an average of 15-20 minutes in research models, while polysomnographic recordings demonstrate increased consolidation of sleep periods with fewer awakenings throughout the night. Importantly, animal models in controlled sleep studies demonstrate improved objective sleep parameters, suggesting that the peptide enhances the restorative aspects of sleep rather than merely extending sleep duration.
The circadian rhythm effects of DSIP extend beyond immediate sleep induction to encompass broader regulation of daily physiological cycles. Research has shown that DSIP administration in animal models can help synchronize disrupted circadian rhythms, with studies demonstrating improved adaptation in experimental protocols involving altered light-dark cycles. The peptide appears to enhance the natural coupling between sleep-wake cycles and other circadian-regulated processes, including body temperature fluctuations, cortisol secretion patterns, and melatonin production. In animal models, chronic DSIP administration helps restore normal circadian patterns in animals with artificially disrupted light-dark cycles, suggesting that the peptide may function as a circadian synchronizing agent. These effects occur without the development of tolerance or dependence that characterizes many conventional sleep medications, making DSIP particularly valuable for research into natural sleep regulation mechanisms and investigational applications for circadian rhythm research.
Neurotransmitter System Interactions and CNS Effects
DSIP's broad spectrum of central nervous system effects reflects its complex interactions with multiple neurotransmitter systems, creating a unique pharmacological profile that distinguishes it from compounds targeting single neurotransmitter pathways. Research has demonstrated that DSIP modulates gamma-aminobutyric acid (GABA) system activity, but through mechanisms distinct from conventional GABA agonists. Studies show that DSIP enhances GABA-A receptor sensitivity without directly binding to benzodiazepine sites, producing anxiolytic effects without the sedation, tolerance, or withdrawal symptoms associated with traditional benzodiazepines. This selective GABA modulation contributes to DSIP's calming effects while preserving cognitive function and natural sleep architecture, a combination rarely achieved by conventional anxiolytic medications.
The serotonergic system represents another major target for DSIP's neuromodulatory effects, with research revealing complex interactions that influence mood, sleep, and pain perception. Laboratory studies demonstrate that DSIP administration increases serotonin turnover in specific brain regions, particularly the hypothalamus and brainstem areas involved in sleep regulation. However, these effects appear to involve modulation of serotonin metabolism rather than direct receptor interactions, as DSIP's effects are not blocked by specific serotonin receptor antagonists. The peptide's influence on serotonin systems may contribute to its mood-stabilizing properties and its efficacy in addiction treatment protocols, where serotonin dysfunction plays a significant role in withdrawal symptoms and relapse susceptibility.
DSIP's interactions with the dopaminergic system provide insights into its effects on reward pathways, motivation, and addiction recovery. Research has shown that DSIP can normalize dopamine receptor sensitivity in experimental models of addiction, potentially explaining its remarkable efficacy in treating withdrawal syndromes. Studies demonstrate that DSIP administration reduces dopamine receptor upregulation that occurs during chronic substance use, while simultaneously protecting against the dopamine depletion that characterizes withdrawal states. These dopaminergic effects may also contribute to DSIP's reported benefits in treating depression and improving cognitive function, as dopamine plays crucial roles in motivation, attention, and executive function. The peptide's ability to modulate multiple neurotransmitter systems simultaneously, without producing the side effects typical of drugs targeting these systems individually, represents a unique pharmacological profile that continues to intrigue neuroscience researchers.
Stress Response and HPA Axis Regulation
One of DSIP's most research-significant properties involves its profound modulation of the hypothalamic-pituitary-adrenal (HPA) axis and overall stress response systems in experimental models. Research has consistently demonstrated that DSIP administration produces marked reductions in stress-induced cortisol elevation, with studies showing 40-60% decreases in cortisol levels following acute stress challenges in laboratory animal models. This cortisol-suppressing effect occurs without the immunosuppression or metabolic disruption typically associated with pharmaceutical corticosteroid antagonists, suggesting that DSIP modulates stress responses through natural regulatory mechanisms rather than pharmacological blockade. The peptide's ability to normalize stress responses while preserving adaptive stress reactions in animal models makes it particularly valuable for research into stress-related physiological mechanisms.
The stress-protective effects of DSIP extend beyond simple cortisol reduction to encompass comprehensive regulation of stress-responsive systems throughout the body in experimental models. Studies have shown that DSIP pretreatment significantly reduces the cardiovascular impact of acute stress, with treated animals showing blunted increases in heart rate and blood pressure during stress challenges. The peptide also appears to preserve immune function during chronic stress conditions in research models, preventing the immunosuppression that typically accompanies prolonged cortisol elevation. Research demonstrates that experimental animals receiving DSIP maintain normal immune cell proliferation and cytokine production even under conditions of chronic mild stress that would typically impair immune function in untreated controls.
The mechanisms underlying DSIP's stress-protective effects involve modulation of key stress-responsive neurotransmitter systems and neuroendocrine pathways. Research suggests that DSIP enhances the activity of stress-protective systems while dampening excessive stress responses in animal models, creating a more balanced stress response profile. Studies have shown that DSIP administration increases brain-derived neurotrophic factor (BDNF) expression in stress-sensitive brain regions, potentially explaining its neuroprotective effects during chronic stress exposure in research models. The peptide also appears to modulate hypothalamic corticotropin-releasing hormone (CRH) activity, reducing excessive activation of the HPA axis while preserving appropriate stress responses in experimental systems. These regulatory effects make DSIP valuable for research into stress-related physiological mechanisms and neuroendocrine regulation.
Addiction Treatment and Withdrawal Management Research
A particularly striking discovery in DSIP research has been its effects on addiction-related neurochemical mechanisms in experimental models. Research investigations have explored DSIP's effects on neurotransmitter systems involved in substance dependence using laboratory animal models. Studies in addiction research models suggest interactions with dopaminergic, GABAergic, and opioid systems, offering insights into novel approaches for investigating withdrawal mechanisms. These findings have made DSIP a valuable tool in addiction neuroscience research for studying dependence and recovery processes in controlled laboratory settings.
The mechanisms underlying DSIP's effects in addiction research models appear to involve modulation of the neurochemical disruptions that characterize substance dependence and withdrawal in experimental systems. Research has shown that chronic substance use produces characteristic changes in neurotransmitter receptor sensitivity, particularly involving dopamine, GABA, and opioid systems. DSIP administration in animal models influences receptor function normalization kinetics while affecting withdrawal-associated behaviors. Studies demonstrate that DSIP-treated animals in experimental withdrawal models show altered behavioral responses including modified anxiety-related behaviors and sleep-wake patterns compared to control groups. Importantly, DSIP itself shows no evidence of tolerance, dependence, or abuse potential in research investigations.
Research protocols for investigating withdrawal mechanisms in animal models have been developed over decades of study, with laboratory investigations exploring dosing parameters and administration schedules in experimental systems. Research indicates that DSIP effects are most pronounced when administered during the acute withdrawal phase in animal models, with research protocols varying from 5-14 days depending on experimental parameters and model characteristics. Follow-up studies have shown that experimental models demonstrate altered behavioral patterns and modified neurochemical profiles in withdrawal studies, with effects persisting in long-term assessments. The peptide's effects in research models appear to involve sustained changes in neural function rather than temporary behavioral modifications, explaining the lasting observations in longitudinal studies. These findings have positioned DSIP as a valuable tool in addiction neuroscience research for investigating dependence mechanisms and recovery processes in controlled laboratory environments.
Pain Modulation and Analgesic Properties
DSIP exhibits unique analgesic properties that distinguish it from conventional pain medications through novel, non-opioid mechanisms that provide pain relief without the risks of tolerance, dependence, or respiratory depression. Research has demonstrated that DSIP produces significant analgesia in various experimental pain models, with studies showing 30-50% reductions in pain sensitivity across different types of nociceptive stimuli. Particularly noteworthy is the finding that DSIP's analgesic effects are not blocked by naloxone, the opioid receptor antagonist, indicating that the peptide's pain-relieving properties operate through pathways distinct from traditional opioid mechanisms. This naloxone-insensitive analgesia represents a potentially safer alternative to opioid-based pain management, avoiding the escalating health crisis associated with opioid dependence and overdose.
The pain-modulating mechanisms of DSIP appear to involve multiple levels of nociceptive processing, from peripheral nerve function to central pain processing in the brain and spinal cord. Research has shown that DSIP influences both the transmission of pain signals and the brain's interpretation of those signals, creating comprehensive pain relief that addresses both sensory and affective components of pain experience. Studies demonstrate that DSIP administration reduces activity in pain-processing regions of the brain, including the anterior cingulate cortex and insula, while simultaneously enhancing activity in pain-inhibitory systems. The peptide also appears to modulate inflammatory responses that contribute to pain sensitization, with research showing reduced production of pro-inflammatory cytokines and enhanced resolution of inflammatory processes at injury sites.
Research investigations have explored DSIP's mechanisms in various experimental pain models using laboratory animals. Studies in animal models of chronic pain conditions including neuropathic pain have documented alterations in pain-related behaviors and sleep parameters in experimental subjects. The peptide's ability to influence both pain sensitivity and sleep architecture simultaneously in research models makes it particularly valuable for investigating pain-sleep interactions in controlled laboratory settings. Research has also investigated DSIP's properties in various pain model systems, where its lack of respiratory depression and absence of tolerance development in preclinical studies provide advantages for research applications. These investigations continue in the research phase using animal model systems and in vitro preparations.
Temperature Regulation and Metabolic Effects
DSIP's influence on thermoregulation and metabolic processes represents another fascinating aspect of its broad physiological activity profile. Research has demonstrated that DSIP administration produces significant effects on body temperature regulation, with studies showing enhanced ability to maintain core temperature under challenging thermal conditions. In cold exposure experiments, DSIP-treated animals demonstrate improved cold tolerance and reduced metabolic stress, while heat exposure studies reveal enhanced heat dissipation and reduced thermal strain. These thermoregulatory effects appear to involve modulation of hypothalamic temperature control centers, with research suggesting that DSIP enhances the efficiency of both heat-generating and heat-dissipating mechanisms.
The metabolic effects of DSIP extend beyond temperature regulation to encompass broader aspects of energy metabolism and metabolic homeostasis. Studies have shown that DSIP influences glucose metabolism, with research demonstrating improved glucose tolerance and enhanced insulin sensitivity in experimental models. The peptide appears to promote more efficient cellular energy utilization while reducing metabolic stress markers, creating a metabolic profile consistent with enhanced physiological efficiency. Research has also documented effects on lipid metabolism, with DSIP administration associated with improved fatty acid oxidation and reduced accumulation of metabolic waste products. These metabolic benefits may contribute to the peptide's stress-protective effects and its ability to enhance recovery from various physiological challenges.
The relationship between DSIP's effects on sleep, stress, and metabolism appears to reflect integrated regulation of fundamental physiological processes rather than independent actions on separate systems. Research suggests that DSIP may function as a master regulator of homeostatic processes, coordinating responses across multiple physiological systems to maintain optimal function under varying conditions. Studies have shown that DSIP's metabolic effects are most pronounced during periods of physiological stress, such as sleep deprivation, caloric restriction, or environmental challenges, suggesting that the peptide enhances adaptive capacity rather than simply altering baseline metabolic function. This integrative activity profile has led researchers to propose that DSIP may represent a fundamental homeostatic signal that has evolved to coordinate complex physiological responses to environmental challenges, though the specific mechanisms underlying these coordinated effects remain to be elucidated.
Immune System Modulation and Neuroprotection
Research into DSIP's effects on immune function has revealed sophisticated immunomodulatory properties that contribute to the peptide's broad therapeutic potential. Studies have demonstrated that DSIP can enhance immune system function while simultaneously preventing excessive inflammatory responses, creating a balanced immune profile that supports host defense without promoting autoimmune or inflammatory pathology. Laboratory investigations show that DSIP administration increases natural killer (NK) cell activity by 25-40% while simultaneously reducing pro-inflammatory cytokine production, including interleukin-1β and tumor necrosis factor-α. This dual effect of immune enhancement and inflammation control represents an ideal therapeutic profile for conditions where immune dysfunction contributes to pathology.
The immunomodulatory mechanisms of DSIP appear to involve regulation of key immune signaling pathways and neuroimmune interactions. Research has shown that DSIP influences the hypothalamic-pituitary-adrenal axis in ways that support optimal immune function, preventing both immunosuppression associated with chronic stress and excessive inflammatory responses that can cause tissue damage. Studies demonstrate that DSIP administration helps maintain normal immune cell proliferation and antibody production even under conditions of chronic stress that would typically impair immune function. The peptide also appears to enhance the resolution of inflammatory processes, promoting the transition from active inflammation to tissue repair and homeostasis restoration.
DSIP's neuroprotective properties have emerged as an important area of research, with studies suggesting potential applications in neurodegenerative diseases and acute brain injury. Research has shown that DSIP can protect neurons against various forms of damage, including oxidative stress, excitotoxicity, and ischemic injury. Laboratory studies demonstrate that DSIP-treated neuronal cultures show 40-60% improved survival under stress conditions compared to untreated controls. The neuroprotective mechanisms appear to involve enhancement of cellular antioxidant systems, improved mitochondrial function, and activation of neuroprotective signaling pathways. Research has also suggested that DSIP may promote neuroplasticity and support learning and memory processes, though these effects require further investigation. The combination of neuroprotective, immunomodulatory, and stress-protective effects has led researchers to investigate DSIP's potential in treating neurodegenerative diseases, traumatic brain injury, and other conditions where neuroinflammation and stress contribute to pathology.
Research Applications and Future Directions
DSIP's unique properties and mysterious mechanisms of action have established it as an invaluable research tool for investigating fundamental questions in neuroscience, sleep medicine, addiction research, and stress physiology. The peptide's ability to modulate multiple physiological systems without identified receptors or genetic basis makes it a singular compound for studying non-traditional signaling mechanisms and systemic physiological integration. Current research applications span diverse fields including chronobiology, where DSIP serves as a tool for investigating circadian rhythm regulation and sleep-wake cycle disorders. The peptide's stress-protective effects make it valuable for research into resilience mechanisms and the physiological basis of stress-related disorders, while its addiction treatment properties provide insights into the neurochemical basis of dependence and recovery.
Emerging research directions are exploring novel applications of DSIP in areas such as aging research, where the peptide's multiple homeostatic effects may provide insights into the mechanisms of healthy aging and age-related decline. Preliminary studies suggest that DSIP's effects on sleep quality, stress resilience, and immune function may contribute to longevity and healthspan, making it an important compound for gerontological research. Investigators are also exploring DSIP's potential in treating neurodevelopmental disorders, where its effects on sleep architecture and stress response may benefit conditions such as autism spectrum disorders and attention-deficit hyperactivity disorder. The peptide's unique immunomodulatory properties are being investigated for applications in autoimmune diseases and cancer research, where balanced immune function is crucial for therapeutic success.
The future of DSIP research is likely to be revolutionized by advances in molecular biology and systems neuroscience that may finally solve the mystery of its mechanism of action. Modern techniques such as single-cell RNA sequencing, advanced proteomics, and systems biology approaches offer new possibilities for identifying DSIP's cellular targets and signaling pathways. Researchers are also investigating the potential for developing DSIP analogs with improved stability, specificity, or therapeutic profiles, though such efforts are complicated by the unknown structure-activity relationships for this peptide. The integration of DSIP research with advances in sleep medicine, addiction treatment, and stress physiology promises to yield new insights into fundamental aspects of mammalian health and disease. As one of neuroscience's greatest remaining mysteries, DSIP continues to challenge our understanding of peptide signaling while offering unprecedented opportunities for therapeutic development and basic scientific discovery.
Conclusion
DSIP stands as one of the most fascinating and enigmatic compounds in modern neuroscience, representing a unique intersection of demonstrable biological activity and fundamental scientific mystery. Nearly five decades after its discovery, this remarkable nonapeptide continues to challenge our understanding of how biological systems function while simultaneously providing invaluable insights into sleep regulation, stress response, addiction treatment, and homeostatic control mechanisms. The peptide's unprecedented breadth of physiological effects, combined with the complete absence of identified receptors or genetic basis, makes DSIP a singular compound that defies conventional categorization and forces researchers to consider novel paradigms for understanding biological signaling and regulation.
The scientific value of DSIP extends far beyond its immediate research applications to encompass broader questions about the nature of biological regulation and the limits of current scientific knowledge. As a compound that demonstrates profound biological activity through completely unknown mechanisms, DSIP serves as a humbling reminder that significant gaps remain in our understanding of fundamental biological processes. For researchers, DSIP represents both a powerful investigational tool and a compelling scientific challenge that promises to yield new insights into the complex relationships between sleep, stress, addiction, and overall physiological homeostasis. The ongoing investigation of DSIP's properties and mechanisms continues to push the boundaries of neuroscience research while offering hope for novel therapeutic approaches to some of medicine's most challenging problems.
References
- Monnier, M. et al. (1977). Functions of the nervous system during sleep—humoral regulation of sleep. Experientia 33(9):1119-1123. [doi.org]
- Schoenenberger, G.A. et al. (1977). The delta EEG (sleep)-inducing peptide (DSIP). XI. Amino-acid analysis, sequence, synthesis and activity of the nonapeptide. Pflügers Archiv European Journal of Physiology 369(1-2):99-109. [PubMed]
- Idzikowski, C. et al. (1984). Delta-sleep-inducing peptide (DSIP): a review. Neuroscience & Biobehavioral Reviews 8(1):83-97. [PubMed]
- Schneider-Helmert, D. et al. (1986). Delta-sleep-inducing peptide (DSIP): an update. Peptides 7(Suppl 1):119-130. [PubMed]
- Tobler, I. et al. (1988). DSIP--a tool for investigating the sleep onset mechanism: a review. Sleep 11(5):459-479. [PubMed]
- Schneider-Helmert, D. et al. (1981). Acute and delayed effects of DSIP (delta sleep-inducing peptide) on human sleep behavior. Peptides 2 Suppl 2:139-146. [PubMed]
- Kovalzon, V.M. et al. (2006). Delta sleep-inducing peptide (DSIP): a still unresolved riddle. Journal of Neurochemistry 97(2):303-309. [PubMed]
- Yehuda, S. et al. (1987). The effects of DSIP on pain threshold during light and dark periods in rats are not naloxone-sensitive. International Journal of Neuroscience 37(1-2):73-76. [PubMed]
- Graf, M.V. et al. (1984). Therapeutic effects of delta-sleep-inducing peptide (DSIP) in patients with chronic, pronounced pain episodes. A clinical pilot study. International Journal of Clinical Pharmacology Research 4(2):139-143. [PubMed]
- Gallimberti, L. et al. (1989). DSIP in the treatment of withdrawal syndromes from alcohol and opiates. European Neuropsychopharmacology 1(3):281-285. [PubMed]
- Danguir, J. et al. (1983). Successful treatment of withdrawal symptoms with delta sleep-inducing peptide, a neuropeptide with potential agonistic activity on opiate receptors. British Journal of Clinical Pharmacology 16(3):334-335. [PubMed]
- Drucker-Colín, R. et al. (1984). Delta sleep-inducing peptide alters REM sleep in normal subjects. Psychopharmacology 83(4):343-346. [doi.org]
- Kastin, A.J. et al. (1981). Permeability of blood-brain barrier to DSIP peptides. Pharmacology Biochemistry and Behavior 15(6):955-959. [PubMed]
- Wisniewski, K. et al. (1987). Passage of delta sleep-inducing peptide (DSIP) across the blood-cerebrospinal fluid barrier. Pharmacology Biochemistry and Behavior 27(3):479-482. [PubMed]
- Kovalzon, V.M. et al. (1984). DSIP reduces amphetamine-induced hyperthermia in mice. Pharmacology Biochemistry and Behavior 20(2):257-261. [PubMed]
- Nagasaki, H. et al. (1981). Thermoregulatory and locomotor effects of DSIP: paradoxical interaction with d-amphetamine. Peptides 2(4):415-420. [PubMed]
- Kitamura, K. et al. (1993). The effect of delta sleep-inducing peptide (DSIP) on the changes of body (core) temperature induced by serotonergic agonists in rats. Brain Research Bulletin 31(1-2):165-168. [PubMed]
- Naitoh, K. et al. (1984). Synthesis of delta sleep-inducing peptide (DSIP) and its physiological activity. Chemical and Pharmaceutical Bulletin 32(6):2347-2354. [PubMed]
- Bonnet, K.A. et al. (1982). Clinical use of delta sleep-inducing peptide. Psychopharmacology Bulletin 18(4):106-113. [PubMed]
| CAS Number | C35H48N10O15 |
|---|---|
| Molecular Formula | C35H48N10O15 |
| Molecular Weight | 848.8 g/Mol |
| Purity | 99.8% |
| Lot Number | 25052 |
| Quantity | 5mg |
| Sequence | H-DL-Trp-DL-Ala-Gly-Gly-DL-Asp-DL-Ala-DL-Ser-Gly-DL-Glu-OH |
Cosmic Peptides
Certificate of Analysis
Unable to load certificate. Download COA





