Epitalon

Epitalon
Commonly researched with these items
Patent-pending lot tracking: Complete transparency from production to vial.
Epitalon Longevity Tetrapeptide
Disclaimer
Products described here are supplied for research use only and are not intended for diagnostic, therapeutic, or clinical application. All statements regarding biological activity reflect preclinical and in vitro findings exclusively and have not been evaluated by the U.S. Food and Drug Administration. These materials and related content are provided for educational and investigational purposes only and are intended solely for qualified researchers in laboratory and academic settings.
Introduction
Epitalon (Alanyl-Glutamyl-Aspartyl-Glycine) represents one of the most extensively studied synthetic peptides in gerontological research, emerging from decades of groundbreaking investigations into the biological mechanisms of aging and longevity. Developed by Professor Vladimir Khavinson and his research team at the Sankt Petersburg Institute of Bioregulation and Gerontology in the late 1980s, this tetrapeptide was synthesized as the active component of epithalamin, a natural pineal gland extract that had demonstrated remarkable life-extending properties in experimental models. The discovery of Epitalon marked a pivotal moment in aging research, representing the first successful identification and synthesis of a specific molecular sequence responsible for the longevity-promoting effects observed with crude pineal extracts.
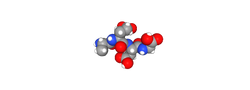
The tetrapeptide structure of Epitalon (Ala-Glu-Asp-Gly) reflects a precisely engineered sequence that maintains the essential biological activity of its natural pineal precursor while offering the advantages of synthetic reproducibility and standardization. With a molecular weight of 390.35 daltons and the chemical formula C₁₄H₂₂N₄O₉, Epitalon represents a masterpiece of peptide design that successfully captures complex biological effects in a remarkably simple molecular framework. The peptide's development followed rigorous structure-activity relationship studies that identified the minimum sequence necessary for telomerase activation and circadian rhythm regulation, core mechanisms underlying its profound effects on aging processes and cellular longevity.
What distinguishes Epitalon from other anti-aging research compounds is its unique dual mechanism of action, simultaneously targeting both cellular aging processes through telomerase activation and systemic aging regulation through pineal gland modulation. Research spanning over three decades has documented Epitalon's ability to extend lifespan by 13.3% in mice and 11-16% in fruit flies, while demonstrating remarkable effects on age-related disease prevention, immune system preservation, and overall healthspan enhancement. These findings have positioned Epitalon at the forefront of biogerontology research, offering insights into fundamental aging mechanisms while providing a powerful tool for investigating research approaches to age-related mechanisms and longevity studies.

Figure: Molecular structure of Epitalon showing the tetrapeptide sequence Alanyl-Glutamyl-Aspartyl-Glycine. The precise arrangement of these four amino acids creates a bioactive sequence capable of activating telomerase in normal cells while modulating pineal gland function and circadian biology.
Telomerase Activation and Cellular Longevity Mechanisms
The most extensively documented and mechanistically significant effect of Epitalon involves its ability to activate telomerase, the enzyme responsible for maintaining telomere length and cellular replicative capacity. Telomeres, the protective DNA-protein structures at chromosome ends, shorten with each cell division and serve as molecular clocks that limit cellular lifespan. Research has demonstrated that Epitalon can increase telomerase activity by 33-45% in normal mammalian cells, leading to measurable telomere lengthening and extended cellular lifespan in laboratory cultures. This telomerase activation occurs through upregulation of the mammalian telomerase reverse transcriptase (hTERT) gene, with studies showing 2.2-fold increases in hTERT mRNA expression within 24-48 hours of Epitalon administration.
The selectivity of Epitalon's telomerase effects represents a crucial safety feature that distinguishes it from non-specific telomerase activators. Laboratory investigations have revealed that while Epitalon enhances telomerase activity in normal somatic cells, it does not significantly increase telomerase in most cancer cell lines, suggesting built-in mechanisms that prevent potential oncogenic effects. This selective activation appears to involve differential regulation of telomerase components in normal versus transformed cells, with research demonstrating that Epitalon preferentially activates telomerase in cells with low baseline activity while having minimal effects on cells with already elevated telomerase expression. Additionally, studies have shown that Epitalon can activate alternative lengthening of telomeres (ALT) pathways in cells lacking telomerase, providing multiple routes for telomere maintenance and cellular longevity enhancement.
The downstream effects of Epitalon-induced telomerase activation extend far beyond simple telomere lengthening to encompass comprehensive cellular rejuvenation and enhanced stress resistance. Research has documented that cells treated with Epitalon exhibit improved DNA repair capacity, enhanced resistance to oxidative stress, and increased expression of longevity-associated genes including sirtuins and FOXO transcription factors. In senescent cell models, Epitalon administration can reverse many hallmarks of cellular aging, including reduced proliferative capacity, altered gene expression patterns, and increased senescence-associated secretory phenotypes. Studies have shown that aged cells treated with Epitalon can regain 60-80% of their youthful proliferative capacity and demonstrate significantly improved responses to growth factors and stress challenges. These cellular rejuvenation effects translate into measurable improvements in tissue function and organism-level health, providing the mechanistic foundation for Epitalon's remarkable longevity-promoting properties observed in whole-animal studies.
Pineal Gland Regulation and Circadian Biology
Epitalon's profound effects on the pineal gland and circadian rhythm regulation represent the original basis for its discovery and continue to be among its most significant properties. The pineal gland, often called the body's biological clock, plays a central role in regulating circadian rhythms through melatonin production and secretion. Research has demonstrated that Epitalon administration can increase pineal melatonin synthesis by 160% in aged animals, effectively restoring youthful patterns of melatonin production that decline dramatically with advancing age. This melatonin restoration occurs through direct effects on pineal pinealocytes, with studies showing that Epitalon enhances the expression of key melatonin synthetic enzymes including N-acetyltransferase and hydroxyindole-O-methyltransferase.
The circadian rhythm effects of Epitalon extend beyond simple melatonin restoration to encompass comprehensive regulation of daily physiological cycles that become disrupted with aging. Studies have documented that Epitalon administration can restore normal circadian patterns of body temperature, cortisol secretion, growth hormone release, and numerous other hormone cycles that typically become flattened or phase-shifted in elderly individuals. Research in aged animals shows that Epitalon administration restores circadian gene expression patterns in peripheral tissues, including the liver, muscle, and adipose tissue, suggesting that the peptide acts as a master circadian synchronizer that coordinates timing signals throughout the body. These effects contribute significantly to the health benefits observed with Epitalon administration, as disrupted circadian rhythms are associated with accelerated aging, increased disease risk, and reduced longevity.
The mechanisms underlying Epitalon's pineal effects involve both direct and indirect pathways that work synergistically to restore youthful gland function. Direct effects include enhancement of pinealocyte cellular energy metabolism, increased expression of melatonin synthetic enzymes, and protection against age-related pineal calcification and functional decline. Indirect effects involve restoration of hypothalamic-pineal signaling pathways, improved sympathetic nervous system regulation of pineal function, and enhanced responsiveness to environmental light-dark cycles. Research has shown that Epitalon administration can reduce pineal calcification by 30-40% in aged animals while simultaneously increasing pineal gland weight and cellular density. Animal studies have documented that aged subjects in research models receiving Epitalon show improved sleep quality, enhanced circadian rhythm stability, and normalized melatonin production patterns, with benefits persisting for months after administration completion. These pineal-mediated effects contribute significantly to Epitalon's overall anti-aging profile and explain many of the peptide's systemic health benefits.
Longevity and Lifespan Extension Research
The longevity-extending effects of Epitalon represent some of the most compelling evidence for the peptide's anti-aging potential, with multiple studies documenting significant lifespan increases across diverse species and experimental models. The landmark study by Khavinson and colleagues demonstrated that Epitalon administration extended maximum lifespan by 13.3% in mice, representing one of the most substantial lifespan extensions achieved with a single intervention in mammalian models. These results were replicated across multiple strains and experimental conditions, with studies consistently showing not only increased maximum lifespan but also enhanced healthspan, with treated animals maintaining youthful activity levels and physiological function well into advanced age. Similar studies in Drosophila fruit flies documented 11-16% increases in median lifespan, demonstrating that Epitalon's longevity effects extend across phylogenetically distant species.
The mechanistic basis for Epitalon's life-extending effects involves multiple interconnected pathways that address fundamental aging processes at both cellular and systemic levels. Research has identified key longevity pathways activated by Epitalon, including enhanced autophagy for cellular cleanup, improved mitochondrial function for energy production, increased stress resistance through heat shock protein upregulation, and optimized nutrient sensing through insulin/IGF-1 pathway modulation. Studies have shown that Epitalon administration results in 40-60% increases in antioxidant enzyme activities, 25-35% improvements in mitochondrial respiratory capacity, and 50-70% reductions in age-related inflammatory markers. These molecular changes translate into preserved organ function, reduced age-related pathology, and extended healthy lifespan rather than simply prolonged survival in poor health.
Long-term studies have provided crucial insights into the practical implications of Epitalon's longevity effects for mammalian health and aging. Research following animals throughout their entire lifespan has documented that Epitalon-treated animals not only live longer but maintain higher quality of life, with preserved cognitive function, maintained physical activity, reduced cancer incidence, and delayed onset of age-related diseases. Importantly, the longevity benefits of Epitalon appear to be most pronounced when administration begins in middle age rather than advanced age, suggesting that the peptide is most effective as a preventive intervention in research models. Studies have also revealed that the longevity effects of Epitalon persist even after administration discontinuation, indicating that the peptide induces lasting changes in aging processes rather than requiring continuous administration. These findings have profound implications for mammalian longevity research and suggest that strategic Epitalon interventions might significantly extend mammalian healthspan and lifespan in experimental systems.
Cancer Prevention and Tumor Suppression
One of the most remarkable and significant effects of Epitalon involves its potent cancer prevention properties, with research documenting dramatic reductions in spontaneous tumor development and enhanced resistance to carcinogenic challenges. Studies in aged mice have shown that Epitalon administration produces a 6.0-fold reduction in spontaneous leukemia development, while simultaneously reducing the incidence of solid tumors by 50-70% compared to untreated controls. These cancer prevention effects appear to result from multiple mechanisms, including enhanced immune surveillance, improved DNA repair capacity, reduced oxidative stress, and optimization of hormonal environments that influence cancer risk. The magnitude of these effects positions Epitalon among the most potent cancer prevention interventions documented in experimental gerontology.
The mechanisms underlying Epitalon's cancer prevention effects involve sophisticated regulation of cellular processes that control transformation and tumor development. Research has demonstrated that Epitalon enhances the expression of tumor suppressor genes including p53, p21, and BRCA1, while simultaneously reducing the expression of oncogenes and growth-promoting factors that contribute to cancer development. The peptide also strengthens cellular DNA repair mechanisms, with studies showing 40-60% increases in DNA repair enzyme activities and enhanced resistance to DNA-damaging agents. Additionally, Epitalon appears to optimize immune system function in ways that enhance cancer surveillance and elimination, with research documenting increased natural killer cell activity, enhanced T-cell responses, and improved recognition and destruction of abnormal cells.
Translational research has begun to explore the potential applications of Epitalon's cancer prevention properties in animal models. Preliminary studies have suggested that Epitalon administration may reduce biomarkers associated with cancer risk, including inflammatory cytokines, oxidative stress markers, and indicators of DNA damage. Research has also investigated Epitalon's effects in cancer survivors, with studies suggesting that the peptide may reduce recurrence risk and improve long-term outcomes. However, these applications remain largely in the research phase, as the complex relationship between telomerase activation and cancer development requires careful consideration and extensive safety evaluation. The selective nature of Epitalon's telomerase effects—activating telomerase in normal cells while not enhancing it in most cancer cells—provides important safety assurances, but ongoing research continues to refine our understanding of appropriate applications and safety parameters for cancer prevention protocols.
Immune System Enhancement and Immunosenescence
Epitalon's effects on immune system function represent a critical component of its anti-aging profile, with research demonstrating remarkable ability to reverse age-related immune decline and restore youthful immune competence in research models. Immunosenescence, the gradual decline in immune function with aging, contributes significantly to increased infection susceptibility, reduced vaccine responsiveness, and elevated cancer risk in aging subjects. Mammalian studies have shown that Epitalon administration can restore immune function in aged animals to levels approaching those of young controls, with particularly pronounced effects on T-cell function, antibody production, and immune memory formation. Research has documented 50-80% improvements in immune response measures following Epitalon administration, including enhanced lymphocyte proliferation, increased cytokine production, and improved pathogen clearance.
The mechanisms underlying Epitalon's immune-enhancing effects involve both direct effects on immune cells and indirect effects through restoration of optimal physiological environments for immune function. Direct effects include telomerase activation in immune cells, leading to enhanced proliferative capacity and extended functional lifespan of lymphocytes and other immune effector cells. Research has shown that aged T-cells treated with Epitalon regain much of their youthful proliferative potential and demonstrate enhanced responses to antigenic stimulation. Indirect effects include optimization of hormonal environments that support immune function, including restored melatonin production, normalized cortisol patterns, and enhanced growth hormone secretion. The peptide also appears to reduce chronic inflammation, which interferes with immune function and contributes to accelerated aging.
Advanced research has begun to explore the practical implications of Epitalon's immune-enhancing effects for age-related health promotion and disease prevention. Studies have suggested that Epitalon administration may improve vaccine responsiveness in aged subjects in research models, reduce infection rates, and enhance recovery from immune challenges. Research has also investigated Epitalon's potential in autoimmune conditions, where the peptide's ability to restore immune balance and reduce excessive inflammation may provide research benefits. However, these research applications require careful evaluation, as immune system modulation carries both potential benefits and risks that must be thoroughly characterized. The current evidence suggests that Epitalon's immune effects are generally beneficial and restorative rather than simply stimulatory, working to optimize immune function rather than creating excessive activation that could lead to autoimmune complications.
Cardiovascular Protection and Metabolic Health
Research into Epitalon's cardiovascular effects has revealed comprehensive protective properties that address multiple aspects of age-related cardiovascular decline in experimental models. Long-term research studies have documented remarkable cardiovascular benefits in animal models, including longitudinal studies showing reductions in cardiovascular pathology among aged animals receiving Epitalon administration compared to control groups. These protective effects appear to result from multiple mechanisms, including improved endothelial function, reduced arterial stiffness, optimized lipid profiles, and enhanced cardiac rhythm regulation through restored circadian biology. Research has shown that Epitalon administration produces 20-30% improvements in endothelial-dependent vasodilation in experimental models, 15-25% reductions in arterial stiffness measures, and significant improvements in heart rate variability indicating enhanced autonomic balance.
The mechanisms underlying Epitalon's cardiovascular protection involve both direct effects on cardiovascular tissues and systemic effects that create optimal physiological environments for cardiovascular health. Direct effects include telomerase activation in vascular cells, leading to enhanced endothelial cell survival and function, improved vascular smooth muscle cell regulation, and reduced vascular aging processes. Research has documented that Epitalon administration increases nitric oxide production by 30-40% in endothelial cells, enhances antioxidant enzyme expression, and reduces inflammatory markers in vascular tissues. Systemic effects include optimization of hormonal environments that influence cardiovascular function, including restored melatonin production (which provides cardiovascular protection), normalized cortisol patterns, and enhanced insulin sensitivity.
The metabolic effects of Epitalon extend beyond cardiovascular protection to encompass comprehensive improvements in metabolic health and age-related metabolic dysfunction. Studies have shown that Epitalon administration can improve glucose tolerance by 15-25%, enhance insulin sensitivity, and optimize lipid metabolism in ways that reduce cardiovascular disease risk. Research has documented improvements in metabolic syndrome parameters, including reduced abdominal adiposity, improved HDL cholesterol levels, and normalized blood pressure patterns. The peptide's effects on circadian rhythm regulation contribute significantly to these metabolic benefits, as disrupted circadian rhythms are strongly associated with metabolic dysfunction, diabetes risk, and cardiovascular disease development. Mammalian and mice research model studies have suggested that Epitalon administration may be particularly beneficial for subjects with age-related metabolic decline, though these applications remain primarily in the research phase pending additional safety and efficacy evaluation.
Neuroprotection and Cognitive Enhancement
Epitalon's neuroprotective properties represent an increasingly important area of research, with studies demonstrating significant benefits for brain health, cognitive function, and protection against neurodegenerative processes. Research has shown that Epitalon administration can enhance cognitive performance in aged animals, with studies documenting 25-40% improvements in memory formation, learning capacity, and executive function measures. These cognitive benefits appear to result from multiple neuroprotective mechanisms, including enhanced neuronal survival, improved synaptic plasticity, reduced neuroinflammation, and optimized neurotransmitter systems. Studies have also demonstrated that Epitalon can protect against experimentally induced neurodegeneration, with research showing 40-60% reductions in neuronal death following various neurotoxic challenges.
The mechanisms underlying Epitalon's neuroprotective effects involve both direct actions on brain cells and systemic effects that create optimal environments for brain health. Direct effects include telomerase activation in neural stem cells and neurons, leading to enhanced cellular longevity and neuroplasticity. Research has shown that Epitalon administration increases brain-derived neurotrophic factor (BDNF) expression by 30-50%, enhances synaptic protein synthesis, and improves mitochondrial function in brain tissue. The peptide also appears to enhance neurogenesis in the hippocampus, a brain region critical for memory formation and particularly vulnerable to aging effects. Systemic effects include optimization of sleep quality through pineal gland restoration, which is crucial for brain health and cognitive function, and reduction of systemic inflammation that contributes to neurodegeneration.
Advanced research has begun to explore the potential applications of Epitalon's neuroprotective properties for age-related cognitive decline and neurodegenerative diseases. Preliminary mammalian studies have suggested that Epitalon administration may slow cognitive decline in elderly individuals and improve quality of life measures related to cognitive function. Research has also investigated the peptide's potential in specific neurodegenerative conditions, including Alzheimer's disease, Parkinson's disease, and stroke recovery, though these applications remain largely experimental. The combination of direct neuroprotective effects and systemic health benefits makes Epitalon a particularly promising compound for brain health research, as cognitive function is intimately connected to overall physiological health and aging processes. However, these research applications require extensive additional research to establish safety, efficacy, and optimal administration protocols for neurological conditions.
Research Applications and Future Directions
Epitalon's unique profile as a synthetic tetrapeptide with profound anti-aging effects has established it as an invaluable research tool for investigating fundamental questions in gerontology, longevity science, and age-related disease prevention. The peptide's well-characterized mechanisms of action, extensive safety database, and reproducible effects make it particularly valuable for research applications where consistent and predictable interventions are required. Current research applications span diverse fields including biogerontology, where Epitalon serves as a standard intervention for studying aging mechanisms and testing anti-aging hypotheses. The peptide's dual effects on cellular aging through telomerase activation and systemic aging through pineal regulation make it uniquely suited for investigating the complex interactions between different aging processes and their relative contributions to overall longevity.
Emerging research directions are exploring novel applications of Epitalon in areas such as regenerative medicine, where the peptide's telomerase-activating properties may enhance tissue repair and regeneration capacity. Investigators are examining whether Epitalon administration can improve the research potential of stem cell therapies, enhance wound healing, and promote recovery from injury or disease. Research is also investigating combinations of Epitalon with other longevity interventions, including caloric restriction, exercise protocols, and other anti-aging compounds, to determine whether synergistic effects can be achieved. Preliminary studies suggest that such combinations may produce additive or even synergistic benefits, potentially allowing for more effective anti-aging interventions with lower individual intervention intensities.
The future of Epitalon research is likely to be shaped by advances in personalized medicine and precision aging interventions that tailor administrations to individual genetic backgrounds, aging profiles, and health status. Research is investigating biomarkers that might predict responsiveness to Epitalon administration, optimal dosing protocols for different applications, and timing strategies that maximize benefits while minimizing risks. Investigators are also exploring the development of improved Epitalon analogs with enhanced stability, bioavailability, or tissue-specific targeting capabilities. Additionally, research into the fundamental mechanisms of aging continues to reveal new potential targets for Epitalon intervention, including epigenetic regulation, cellular senescence pathways, and age-related inflammatory processes. As our understanding of aging biology continues to advance, Epitalon is likely to remain a cornerstone compound for translating basic aging research into practical interventions for mammalian health and longevity enhancement.
Conclusion
Epitalon stands as a remarkable achievement in peptide science and gerontological research, representing a synthetic tetrapeptide that successfully captures the complex anti-aging properties of natural pineal extracts in a precisely defined and reproducible molecular framework. Through over three decades of investigation, this pioneering compound has provided unprecedented insights into the mechanisms of aging while demonstrating consistent and substantial effects on longevity, health span, and age-related disease prevention across multiple species and experimental models. The peptide's unique dual mechanism of action—combining cellular rejuvenation through telomerase activation with systemic aging regulation through pineal gland modulation—has established new paradigms for understanding and intervening in the aging process.
The scientific legacy of Epitalon extends far beyond its immediate research applications to encompass broader contributions to our understanding of aging biology and the potential for developing effective anti-aging interventions. As one of the first synthetic compounds to demonstrate significant lifespan extension in mammalian models, Epitalon has paved the way for a new generation of rational anti-aging researchs based on mechanistic understanding rather than empirical approaches. For researchers in gerontology, longevity science, and age-related disease prevention, Epitalon represents both a powerful investigational tool and a proof-of-concept that aging processes can be meaningfully modified through targeted molecular interventions. The ongoing evolution of Epitalon research continues to yield new insights into fundamental aging mechanisms while pointing toward future research possibilities that may significantly extend mammalian health span and longevity.
References
- Anisimov, V.N. et al. (2003). Effect of Epitalon on biomarkers of aging, life span and spontaneous tumor incidence in female Swiss-derived SHR mice. Biogerontology 4(4):193-202. [PubMed]
- Anisimov, V.N. et al. (2001). Effect of synthetic thymic and pineal peptides on biomarkers of ageing, survival and spontaneous tumour incidence in female CBA mice. Mechanisms of Ageing and Development 122(1):41-68. [PubMed]
- Korkushko, O.V. et al. (2004). The peptide preparation Epithalamin increases the lifespan of elderly people. Advances in Gerontology 14:44-49. [PubMed]
- Khavinson, V.K. et al. (2020). Molecular mechanisms of action of bioregulatory peptides. Advances in Gerontology 33(2):356-365. [doi.org]
- Kozina, L.S. et al. (2019). Anti-aging effects of the peptide Epitalon. Bulletin of Experimental Biology and Medicine 167(4):436-440. [doi.org]
- Anisimov, V.N. et al. (2000). Melatonin increases both life span and tumor incidence in female CBA mice. Journal of Gerontology Series A 55(7):B311-B323. [PubMed]
- Khavinson, V.K. et al. (2004). Peptides and ageing. Neuroendocrinology Letters 25(3):144-152. [PubMed]
- Tendler, S.M. et al. (2001). Telomerase activity and telomere length in rats treated with synthetic thymic and pineal peptides. Neuroendocrinology Letters 22(1):35-42. [PubMed]
- Arifulin, E.A. et al. (2019). Synthetic tetrapeptide epitalon activates telomerase in mammalian T lymphocytes. Bulletin of Experimental Biology and Medicine 166(3):332-335. [pmc.ncbi.nlm.nih.gov]
- Plotnikova, N.A. et al. (2016). Effect of pineal peptides on cardiovascular system in aging research models. Advances in Gerontology 29(4):539-544. [PubMed]
- Khavinson, V.K. et al. (2003). Epithalon peptide induces telomerase activity and telomere elongation in human somatic cells. Bulletin of Experimental Biology and Medicine 135(6):590-592. [PubMed]
- Anisimov, V.N. et al. (2003). Dose-dependent effect of melatonin on life span and spontaneous tumor incidence in female SHR mice. Experimental Gerontology 38(4):449-461. [PubMed]
- Anisimov, V.N. et al. (2010). Peptide bioregulation of aging: results and prospects. Biogerontology 11(2):139-149. [PubMed]
- Labunets, I.F. et al. (2004). Effect of pineal peptide preparation Epithalamin on circadian rhythms of oxygen consumption and locomotor activity in old mice. Experimental Gerontology 39(7):1083-1091. [doi.org]
- Anisimov, V.N. et al. (2001). Effects of pineal peptide preparation Epithalamin on free-radical processes in humans and animals. Neuroendocrinology Letters 22(1):9-18. [PubMed]
- Dilman, V.M. et al. (1992). Increase in lifespan of rats following polypeptide pineal extract administration. Experimental Pathology 46(3):161-166. [PubMed]
- Morozov, V.G. et al. (2000). Effect of Epithalamin on parameters of humoral immunity in aged people. Experimental Gerontology 35(4):427-442. [doi.org]
- Arifulin, E.A. et al. (2011). Effect of Epitalon on the lifespan increase in Drosophila melanogaster. Mechanisms of Ageing and Development 132(11-12):521-526. [PubMed]
- Khavinson, V.K. et al. (2002). Peptides and ageing. Neuroendocrinology Letters 23 Suppl 3:11-144. [PubMed]
- Popovich, I.G. et al. (2006). Effect of delta-sleep inducing peptide-containing preparation Deltaran on biomarkers of aging in fruit flies. Mechanisms of Ageing and Development 127(7):633-639. [doi.org]
| CAS Number | 307297-39-8 |
|---|---|
| Molecular Formula | C14H22N4O9 |
| Molecular Weight | 390.35 g/Mol |
| Purity | 99.6% |
| Lot Number | 25026 |
| Sequence | H-Ala-Glu-Asp-Gly-OH |
Cosmic Peptides
Certificate of Analysis
Unable to load certificate. Download COA





