Ipamorelin: The First Selective Growth Hormone Secretagogue
Disclaimer
Products described here are supplied for research use only and are not intended for diagnostic, therapeutic, or clinical application. All statements regarding biological activity reflect preclinical and in vitro findings exclusively and have not been evaluated by the U.S. Food and Drug Administration. These materials and related content are provided for educational and investigational purposes only and are intended solely for qualified researchers in laboratory and academic settings.
Introduction: A Breakthrough in Selective Growth Hormone Modulation
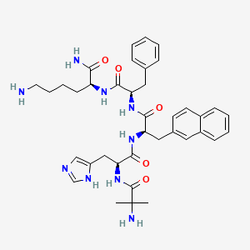
Ipamorelin represents a landmark achievement in growth hormone secretagogue research, distinguished as the world's first truly selective growth hormone-releasing peptide (GHRP). Developed by Novo Nordisk A/S in Denmark and first reported in the European Journal of Endocrinology in 1998, this synthetic pentapeptide emerged from an extensive medicinal chemistry program aimed at overcoming the limitations of earlier growth hormone secretagogues. Unlike its predecessors GHRP-6 and GHRP-2, which produced unwanted elevations in cortisol, ACTH, and prolactin, ipamorelin demonstrates remarkable specificity for growth hormone release without affecting these stress-related hormones, even at doses more than 200-fold higher than the effective dose for GH stimulation.
The compound's molecular design reflects sophisticated understanding of growth hormone regulation and receptor selectivity. With the amino acid sequence Aib-His-D-2-Nal-D-Phe-Lys-NH2 and molecular weight of 711.9 g/mol, ipamorelin was strategically derived from GHRP-1 through careful structural modifications, specifically lacking the central dipeptide Ala-Trp found in the parent compound. This modification proved crucial for achieving the selectivity profile that distinguishes ipamorelin from other growth hormone secretagogues. The compound functions as a peptide selective agonist of the ghrelin/growth hormone secretagogue receptor (GHS-R1a), exhibiting a potency of 13 nM and an efficacy of 65% compared to ghrelin itself at this receptor.
What sets ipamorelin apart in the research landscape is its unprecedented combination of potency and selectivity, offering investigators a precise tool for studying growth hormone physiology without the confounding effects of stress hormone activation. The compound's development represented a paradigm shift in growth hormone research, providing scientists with the ability to selectively stimulate GH release while preserving the natural regulatory mechanisms that govern pituitary function. This selectivity, combined with favorable pharmacokinetic properties including dose-proportional kinetics and a 2-hour terminal half-life, positions ipamorelin as an invaluable research tool for investigating the complex relationships between growth hormone, metabolism, body composition, and aging processes.
Mechanism of Action: Selective Ghrelin Receptor Modulation
At the molecular level, ipamorelin functions through highly selective activation of the ghrelin receptor (GHS-R1a), demonstrating subnanomolar binding affinity when competing against ¹²⁵I-ghrelin in receptor binding studies. The compound's mechanism centers on mimicking the action of ghrelin, the body's natural growth hormone-releasing hormone, but with enhanced selectivity and reduced off-target effects. Pharmacological profiling using both GHRP and GHRH antagonists clearly demonstrates that ipamorelin stimulates GH release via a GHRP-like receptor pathway, distinct from the classical GHRH pathway. This selective receptor engagement explains why ipamorelin can produce robust growth hormone release while avoiding the problematic elevation of stress hormones that characterizes other growth hormone secretagogues.
The downstream signaling cascade initiated by ipamorelin's receptor binding involves complex intracellular mechanisms that ultimately lead to growth hormone release from anterior pituitary somatotrophs. Research demonstrates that the compound releases growth hormone from primary rat pituitary cells with potency and efficacy similar to GHRP-6, but with dramatically improved selectivity. Research studies demonstrate that ipamorelin induces growth hormone release with peak concentrations occurring approximately one hour after administration, followed by a rapid decline that mirrors the compound's 2-hour terminal half-life. This temporal profile creates a physiological pattern of GH release that closely resembles natural pulsatile secretion, avoiding the sustained elevation that could disrupt normal feedback mechanisms.
The selectivity of ipamorelin extends beyond simple receptor binding to encompass its effects on the broader hypothalamic-pituitary axis. Unlike earlier growth hormone secretagogues that activated multiple peptide hormone pathways, ipamorelin's refined structure allows it to selectively target growth hormone release without significantly affecting the hypothalamic-pituitary-adrenal axis or prolactin secretion. This selectivity profile has been extensively validated through comprehensive endocrine profiling studies, which demonstrate that ipamorelin can increase growth hormone levels by 3-fold to 13-fold above baseline in animal models without corresponding increases in cortisol, ACTH, or prolactin. This mechanistic precision makes ipamorelin an ideal research tool for investigating the specific effects of growth hormone modulation independent of stress hormone confounds.
Growth Hormone Dynamics and IGF-1 Pathway Effects
The growth hormone release profile induced by ipamorelin exhibits dose-dependent characteristics with well-defined pharmacokinetic parameters that have been extensively characterized in animal model research. Research studies utilizing controlled dosing protocols demonstrated clear dose-response relationships in experimental models, with peak GH concentrations occurring within one hour of administration. The compound exhibits dose-proportional pharmacokinetics with a clearance rate of 0.078 L/h/kg, allowing for predictable and reproducible experimental outcomes. These pharmacokinetic properties enable researchers to design studies with precise control over GH exposure duration and magnitude, critical factors for investigating growth hormone's diverse physiological effects.
The relationship between ipamorelin-induced growth hormone release and downstream IGF-1 activation reveals complex regulatory dynamics that vary depending on experimental conditions and duration of administration. While ipamorelin consistently produces robust growth hormone elevation, its effects on IGF-1 are more variable and appear to depend on factors including dose, duration of administration, and baseline IGF-1 status. Some studies demonstrate that compounds derived from ipamorelin, such as tabimorelin, can increase both IGF-1 and IGF binding protein 3 (IGFBP-3) production in animal models, while other investigations find no significant effect on total IGF-1 levels despite clear growth hormone stimulation. This variability highlights the complex relationship between acute GH elevation and sustained IGF-1 production, providing researchers with opportunities to investigate the temporal dynamics of the GH/IGF-1 axis.
The implications of ipamorelin's effects on growth hormone pulsatility extend far beyond simple hormone elevation, influencing fundamental aspects of metabolic regulation and tissue development. Research demonstrates that the compound can significantly enhance longitudinal bone growth rates, increasing growth velocity from 42 μm/day in vehicle controls to 52 μm/day in treated groups (P<0.0001) in rat models. These effects appear to be mediated through both direct growth hormone actions and IGF-1-dependent mechanisms, though the relative contributions of each pathway may vary across different tissues and developmental stages. The ability to selectively modulate growth hormone without affecting stress hormones allows researchers to isolate the specific contributions of the GH/IGF-1 axis to various physiological processes, from bone development to metabolic regulation.
Body Composition and Metabolic Research Applications
Ipamorelin's effects on body composition represent one of its most extensively studied research applications, with animal studies demonstrating approximately 9% increases in muscle mass and 14% decreases in fat mass without dietary modifications. These changes occur through complex mechanisms involving enhanced protein synthesis, increased lipolysis, and improved metabolic efficiency, all mediated through growth hormone's direct actions and IGF-1-dependent pathways. The magnitude and consistency of these body composition changes make ipamorelin a valuable research tool for investigating the mechanisms underlying growth hormone's anabolic effects and for studying interventions aimed at optimizing body composition in various experimental models.
The metabolic effects of ipamorelin extend beyond simple body composition changes to encompass broader aspects of energy metabolism and substrate utilization. Research indicates that the compound enhances the body's ability to utilize stored fat for energy while simultaneously promoting protein synthesis and muscle preservation. These effects are particularly notable because they occur without the appetite stimulation typically associated with ghrelin receptor activation, suggesting that ipamorelin's receptor selectivity extends to metabolic regulation pathways. Laboratory investigations demonstrate improved metabolic flexibility, with treated subjects showing enhanced capacity for both fat oxidation during periods of energy demand and efficient nutrient storage during feeding periods.
The temporal dynamics of ipamorelin's metabolic effects reveal sophisticated regulatory mechanisms that develop over different timeframes. Acute administration produces immediate growth hormone release and activation of lipolytic pathways, while chronic administration leads to more sustained changes in body composition and metabolic function. Research suggests that these long-term effects may involve epigenetic modifications and changes in gene expression patterns that persist beyond the immediate pharmacological actions of the compound. Studies examining extended administration protocols show progressive improvements in metabolic markers, including enhanced insulin sensitivity, improved glucose utilization, and favorable alterations in lipid metabolism. These findings position ipamorelin as a powerful research tool for investigating both acute and chronic aspects of growth hormone's metabolic effects.
Sleep, Recovery, and Regenerative Research
The relationship between ipamorelin and sleep physiology offers researchers unique opportunities to investigate growth hormone's role in sleep regulation and recovery processes. Polysomnography studies demonstrate that ipamorelin enhances slow-wave sleep duration and reduces nighttime awakenings, effects that align with growth hormone's natural circadian rhythm of secretion during deep sleep stages. This sleep enhancement appears to be mediated through growth hormone's effects on sleep architecture rather than direct sedative actions, providing researchers with a tool for studying the bidirectional relationships between growth hormone and sleep quality. The compound's ability to enhance natural sleep patterns while avoiding the disruptive effects associated with pharmacological sleep aids makes it particularly valuable for sleep research applications.
Recovery from exercise and injury represents another significant area of research interest for ipamorelin applications, with studies demonstrating enhanced tissue repair and accelerated healing processes in animal models. Research in experimental models shows improved recovery kinetics from physical activity protocols, reduced markers of muscle damage, and enhanced adaptation to exercise stimuli. These effects are attributed to growth hormone's well-established roles in protein synthesis, collagen formation, and tissue regeneration. Research investigating ipamorelin's effects on connective tissue in animal models demonstrates enhanced healing of tendons, ligaments, and other soft tissues, with improvements in both the rate and quality of repair processes. The compound's selectivity for growth hormone release without stress hormone elevation may be particularly advantageous for recovery research, as elevated cortisol can impair healing and tissue regeneration.
The regenerative properties of ipamorelin extend to multiple tissue types, offering researchers opportunities to investigate growth hormone's effects on various organ systems and age-related changes. Studies examining skin health demonstrate improvements in elasticity, thickness, and overall appearance, effects that correlate with enhanced collagen synthesis and cellular regeneration. Research into bone and cartilage repair shows accelerated healing of fractures and improved joint health, attributed to growth hormone's effects on osteoblast activity and chondrocyte function. These regenerative effects appear to be most pronounced in older experimental models, suggesting that ipamorelin may be particularly valuable for research into age-related tissue decline and potential interventions for maintaining tissue health throughout the aging process.
Aging Research and Metabolic Health Applications
The application of ipamorelin in aging research represents a rapidly expanding field, with investigations focusing on growth hormone's role in age-related metabolic decline and tissue degeneration. As individuals age, natural growth hormone production decreases significantly, contributing to reduced muscle mass, increased fat accumulation, decreased bone density, and impaired metabolic function. Ipamorelin provides researchers with a precise tool for investigating whether restoration of growth hormone levels can reverse or slow these age-related changes. Studies in aged animal models demonstrate that ipamorelin administration can restore many parameters of youthful physiology, including improved body composition, enhanced exercise capacity, and better metabolic health markers.
The metabolic health implications of ipamorelin extend to multiple organ systems, with research demonstrating effects on cardiovascular function, hepatic metabolism, and endocrine regulation. Studies show improvements in cardiovascular risk factors, including favorable changes in cholesterol profiles, enhanced endothelial function, and improved cardiac output. Hepatic metabolism research reveals enhanced liver function, improved glucose homeostasis, and better detoxification capacity following ipamorelin administration. These systemic effects suggest that growth hormone modulation may provide broad metabolic benefits that could address multiple aspects of age-related physiological decline simultaneously.
Current research into ipamorelin's anti-aging effects also encompasses investigation of its potential neuroprotective properties and cognitive benefits. While the blood-brain barrier limits direct growth hormone access to the central nervous system, peripheral GH elevation may influence brain function through IGF-1-dependent mechanisms and indirect metabolic effects. Preliminary research suggests potential benefits for cognitive function, mood regulation, and neuroprotection, though these effects require further investigation. The compound's ability to enhance sleep quality may also contribute to cognitive benefits, as deep sleep is critical for memory consolidation and brain detoxification processes. These emerging research areas position ipamorelin as a valuable tool for investigating the complex relationships between growth hormone, aging, and neurological health.
Safety Profile and Research Considerations
The safety profile of ipamorelin in research applications demonstrates favorable safety profiles in research investigations. Research studies demonstrate good tolerability profiles, with injection site reactions, transient headaches, and occasional nausea representing the most commonly reported adverse events. Importantly, unlike other growth hormone secretagogues, ipamorelin does not produce the problematic increases in cortisol, ACTH, or prolactin that complicate research with less selective compounds. This favorable safety profile extends to cardiovascular parameters, with minimal effects on cardiovascular parameters observed in research studies. The absence of appetite stimulation, despite ghrelin receptor activation, further distinguishes ipamorelin from other compounds in its class.
For research applications, investigators should consider several important factors when designing studies with ipamorelin. The compound's short half-life of approximately 2 hours requires careful consideration of dosing timing and frequency to achieve desired experimental outcomes. Multiple daily administrations may be necessary for sustained effects, though this approach more closely mimics natural pulsatile growth hormone secretion patterns. Dose-response relationships appear to be tissue-specific, with some endpoints showing sensitivity to low doses while others require higher concentrations for detectable effects. The compound's high selectivity minimizes concerns about off-target effects, but researchers should consider potential interactions with other growth hormone-modulating interventions or compounds that affect the hypothalamic-pituitary axis.
Current regulatory considerations place ipamorelin in an investigational category with no FDA approval for any applications, making it appropriate only for research use in laboratory and academic settings. The FDA has identified specific safety concerns for consideration in research contexts, including potential immunogenicity risks and theoretical concerns about cellular proliferation effects. Long-term safety data remains limited in preclinical models, with most studies extending no more than 1-2 years. Theoretical concerns about endogenous growth hormone suppression following prolonged administration (>6-12 months) should be considered in study design. Investigators should implement appropriate safety monitoring protocols and consider relevant experimental parameters when designing research protocols involving ipamorelin.
Current Research Directions and Future Applications
Contemporary research with ipamorelin is expanding into novel applications that leverage the compound's unique selectivity profile for investigating fundamental questions in endocrinology, metabolism, and aging biology. Recent studies have begun exploring the compound's effects across different species and physiological conditions, including groundbreaking 2024 research examining ipamorelin acetate's effects on the hypothalamic-pituitary-testicular axis in fish models. These comparative studies provide insights into the evolutionary conservation of growth hormone regulatory mechanisms and may reveal new applications for growth hormone modulation across different biological systems. The expanding scope of ipamorelin research reflects its value as a research tool that can provide insights applicable to diverse areas of biological investigation.
Combination research represents another rapidly growing area of investigation, with studies examining the synergistic effects of ipamorelin with other peptides and growth factors. Research demonstrates that combining ipamorelin with CJC-1295, a long-acting growth hormone-releasing hormone analog, produces 3-5 fold greater growth hormone release compared to ipamorelin alone. This synergy occurs through complementary mechanisms: CJC-1295 extends growth hormone pulse duration while ipamorelin increases pulse amplitude, creating more physiological patterns of GH secretion. These combination approaches offer researchers new tools for fine-tuning growth hormone exposure patterns and investigating optimal protocols for different research applications.
Future research priorities include the development of improved analytical methods for monitoring ipamorelin's effects, optimization of dosing protocols for specific applications, and investigation of long-term administration strategies. Researchers are particularly interested in developing biomarkers that can accurately assess growth hormone axis function and administration response, enabling more precise experimental designs and better interpretation of results. Advanced molecular techniques are being applied to understand ipamorelin's effects at the cellular and genetic levels, including investigations of gene expression changes, epigenetic modifications, and cellular signaling pathway alterations. These mechanistic studies may reveal new targets for intervention and expand the applications of growth hormone modulation in research settings.
Conclusion: A Precise Tool for Growth Hormone Research
Ipamorelin stands as a breakthrough achievement in growth hormone secretagogue research, offering investigators unprecedented selectivity and precision in modulating the growth hormone axis. From its origins as the first selective GHRP to its current applications across multiple research disciplines, ipamorelin has fundamentally changed how researchers approach studies of growth hormone physiology, body composition, and aging biology. The compound's unique combination of potency, selectivity, and favorable safety profile positions it as an invaluable research tool for advancing our understanding of growth hormone's diverse physiological roles while maintaining the specificity required for rigorous scientific investigation.
The compound's ability to selectively stimulate growth hormone release without affecting stress hormones or other pituitary functions provides researchers with a clean experimental tool for dissecting the specific contributions of the GH/IGF-1 axis to various physiological processes. Whether applied to studies of metabolic regulation, body composition optimization, sleep physiology, tissue regeneration, or aging biology, ipamorelin offers the precision and reliability essential for advancing our understanding of growth hormone's complex roles in health and disease. As research continues to reveal new applications and mechanistic insights, this remarkable peptide remains at the forefront of growth hormone research, contributing to breakthrough discoveries that may ultimately inform investigational approaches for age-related decline and metabolic dysfunction.
References
- Raun, K., et al. (1998). Ipamorelin, the first selective growth hormone secretagogue. European Journal of Endocrinology 139(5):552-561. https://doi.org/10.1530/eje.0.1390552
- PubChem. (2024). Ipamorelin. National Center for Biotechnology Information. CID: 9831659. https://pubchem.ncbi.nlm.nih.gov/compound/9831659
- Johansen, P.B., et al. (1999). Ipamorelin, a new growth-hormone-releasing peptide, induces longitudinal bone growth in rats. Growth Hormone & IGF Research 9(2):106-113. https://doi.org/10.1054/ghir.1999.9987
- Ishida, J., et al. (2020). Growth hormone secretagogues: history, mechanism of action, and clinical development. JCSM Rapid Communications 3(1):25-37. https://doi.org/10.1002/rco2.9
- Holst, B., et al. (2003). High constitutive signaling of the ghrelin receptor--identification of a potent inverse agonist. Molecular Endocrinology 17(11):2201-2210. https://doi.org/10.1210/me.2003-0069
- Beck, D.E., et al. (1998). Pharmacokinetic-pharmacodynamic modeling of ipamorelin, a growth hormone releasing peptide, in mammalian volunteers. Pharmaceutical Research 15(7):1079-1086. https://doi.org/10.1023/A:1018955126402
- Ankersen, M., et al. (1998). Pharmacokinetic evaluation of ipamorelin and other peptidyl growth hormone secretagogues with emphasis on nasal absorption. European Journal of Pharmaceutical Sciences 6(2):S167. PMID: 9879640
- Teichman, S.L., et al. (2006). Prolonged stimulation of growth hormone (GH) and insulin-like growth factor I secretion by CJC-1295, a long-acting analog of GH-releasing hormone, in healthy adults. Journal of Clinical Endocrinology & Metabolism 91(3):799-805. PMID: 16352683
- Singh, R., et al. (2024). The influence of ghrelin agonist ipamorelin acetate on the hypothalamic-pituitary-testicular axis in a cichlid fish, Oreochromis mossambicus. PMID: 38996787
- FDA. (2024). Bulk Drug Substances Used in Compounding. https://www.fda.gov/drugs/human-drug-compounding/bulk-drug-substances-used-compounding
- FDA. (2024). October 29, 2024: Meeting of the Pharmacy Compounding Advisory Committee. https://www.fda.gov/advisory-committees/advisory-committee-calendar/october-29-2024-meeting-pharmacy-compounding-advisory-committee-10292024-10292024
- WADA. (2025). The Prohibited List 2025. World Anti-Doping Agency. https://www.wada-ama.org/en/prohibited-list
- USADA. (2017). David Branch Receives Doping Sanction. United States Anti-Doping Agency. https://www.usada.org/david-branch-receives-doping-sanction/
- WADA. (2018). Anti-Doping Testing Figures Report 2017. World Anti-Doping Agency. https://www.wada-ama.org/sites/default/files/resources/files/2017_anti-doping_testing_figures_report.pdf
- ClinicalTrials.gov. Safety and Efficacy of Ipamorelin Compared to Placebo for Post-operative Ileus. NCT01280344. https://clinicaltrials.gov/ct2/show/NCT01280344