Oxytocin

Oxytocin
Commonly researched with these items
Patent-pending lot tracking: Complete transparency from production to vial.
Oxytocin
Disclaimer
Products described here are supplied for research use only and are not intended for diagnostic, therapeutic, or clinical application. All statements regarding biological activity reflect preclinical and in vitro findings exclusively and have not been evaluated by the U.S. Food and Drug Administration. These materials and related content are provided for educational and investigational purposes only and are intended solely for qualified researchers in laboratory and academic settings.
Introduction
Oxytocin stands as one of the most remarkable and intensively studied neuropeptides in modern neuroscience, earning recognition as both the first peptide hormone ever synthesized and a fundamental mediator of social behavior, attachment, and stress regulation across mammalian species. Originally discovered by Sir Henry Dale in 1906 when posterior pituitary extracts contracted the pregnant cat uterus, this nine-amino acid cyclic peptide derives its name from the Greek words "ωκύς" (swift) and "τόκος" (birth), reflecting its initial identification as a potent stimulator of uterine contractions. The groundbreaking work of Vincent du Vigneaud in the 1950s, which led to the first complete chemical analysis and synthesis of oxytocin, earned him the 1955 Nobel Prize in Chemistry and established oxytocin as a prototype for understanding peptide hormone structure-function relationships. This historic achievement marked the beginning of the modern era of peptide chemistry and laid the foundation for decades of research that has revealed oxytocin's profound influence on social cognition, emotional regulation, and physiological homeostasis.
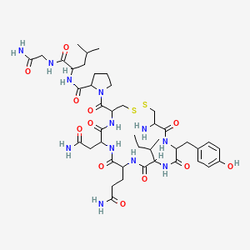
The molecular architecture of oxytocin reflects sophisticated evolutionary design principles that enable its diverse biological activities across multiple physiological systems. This cyclic nonapeptide, with the amino acid sequence Cys–Tyr–Ile–Gln–Asn–Cys–Pro–Leu–Gly–NH₂, contains a critical disulfide bridge between cysteine residues at positions 1 and 6 that creates a six-amino acid ring structure with a three-residue amidated tail. This unique structural organization, shared with the closely related vasopressin (differing by only two amino acids), enables oxytocin to bind with high specificity to its cognate G-protein coupled oxytocin receptor (OXTR) while maintaining sufficient structural flexibility to influence multiple downstream signaling pathways. The evolutionary relationship between oxytocin and vasopressin, which diverged approximately 100 million years ago through gene duplication events, highlights the fundamental importance of these neuropeptides in vertebrate physiology and social organization.
The contemporary research significance of oxytocin extends far beyond its classical reproductive functions to encompass fundamental mechanisms of behavioral neurobiology, stress physiology, and social cognition across mammalian species. Laboratory investigations have revealed oxytocin's central role in regulating social recognition, attachment formation, and stress responses through well-characterized neural pathways conserved across vertebrate evolution. Research demonstrates that oxytocin functions as a social salience enhancer that modulates attention to conspecific cues and influences behavioral responses to social stimuli through distinct neural circuits in the hypothalamus, amygdala, and reward systems. This mechanistic understanding has opened new avenues for investigating the neurobiological basis of social behavior disorders, stress-related pathologies, and emotional dysregulation in laboratory models, while advancing fundamental knowledge of how neuropeptide systems coordinate complex behavioral phenotypes across diverse species and experimental contexts.
Oxytocin Receptor Signaling and Molecular Mechanisms
The biological effects of oxytocin are mediated through its interaction with the oxytocin receptor (OXTR), a seven-transmembrane G-protein coupled receptor that demonstrates sophisticated signaling capabilities and tissue-specific expression patterns throughout the central and peripheral nervous systems. OXTR belongs to the class A rhodopsin-like family of GPCRs and exhibits particularly high expression in key brain regions involved in social behavior and emotional regulation, including the hypothalamus, amygdala, nucleus accumbens, and medial prefrontal cortex. The receptor's distribution pattern provides crucial insights into oxytocin's diverse physiological functions, as OXTR expression in the myometrium and mammary glands underlies its classical reproductive roles, while expression in limbic and cortical regions mediates its effects on social cognition and emotional processing. The tissue-specific regulation of OXTR expression through epigenetic mechanisms, particularly DNA methylation of the receptor gene promoter, contributes to individual differences in oxytocin sensitivity and has been implicated in various psychiatric and developmental conditions.
Upon oxytocin binding, OXTR undergoes conformational changes that activate multiple intracellular signaling cascades, with the primary pathway involving coupling to Gq/11 proteins and subsequent activation of phospholipase C-β (PLC-β). This classical signaling cascade leads to the hydrolysis of phosphatidylinositol 4,5-bisphosphate (PIP₂) into two critical second messengers: diacylglycerol (DAG) and inositol 1,4,5-trisphosphate (IP₃). IP₃ binding to its receptors on the endoplasmic reticulum triggers rapid release of calcium ions into the cytoplasm, while DAG, in combination with elevated calcium levels and phosphatidylserine, activates protein kinase C (PKC). This coordinated calcium-PKC signaling system drives numerous downstream cellular responses including changes in gene expression, synaptic plasticity, and cellular excitability that underlie oxytocin's behavioral and physiological effects. Research has also identified alternative signaling pathways involving Gi/Go coupling that can inhibit adenylyl cyclase and reduce cAMP levels, contributing to the context-dependent nature of oxytocin's effects and providing additional regulatory mechanisms for fine-tuning cellular responses.
The calcium signaling mechanisms activated by oxytocin demonstrate particular complexity and physiological importance, as calcium serves as a universal second messenger that coordinates diverse cellular processes from neurotransmitter release to gene transcription. Beyond the initial IP₃-mediated calcium release from intracellular stores, oxytocin receptor activation can stimulate calcium entry through voltage-gated L-type calcium channels and transient receptor potential (TRP) proteins that mediate store-operated calcium entry. This sustained elevation of intracellular calcium activates calmodulin-dependent protein kinases and phosphatases that regulate synaptic function and neuroplasticity, while also triggering calcium-dependent gene expression programs through transcription factors such as CREB (cAMP response element-binding protein). In peripheral tissues, particularly smooth muscle cells of the uterus and mammary glands, calcium signaling drives the contractile responses that mediate labor induction and milk ejection, demonstrating how the same fundamental signaling mechanisms can produce tissue-specific functional outcomes depending on the cellular context and downstream effector systems present.
Central Nervous System Actions and Blood-Brain Barrier Transport
The central nervous system actions of oxytocin represent one of the most fascinating aspects of its biology, as this neuropeptide must navigate the complex barrier systems that separate peripheral circulation from brain tissue to exert its effects on social behavior and emotional processing. The blood-brain barrier presents a significant challenge for peptide hormones like oxytocin, which has a molecular weight of approximately 1007 Da and limited lipophilicity that restricts passive diffusion across cerebral capillaries. Research has revealed that oxytocin crosses the blood-brain barrier through a saturable transport mechanism mediated by the receptor for advanced glycation end-products (RAGE), achieving a permeability coefficient of approximately 3.0 × 10⁻⁶ cm/s. However, this transport system is relatively inefficient, with only 1-2% of peripherally administered oxytocin reaching the central nervous system, highlighting research interest in alternative delivery methods that can bypass the blood-brain barrier and directly access central nervous system compartments in experimental models.
Within the central nervous system, oxytocin functions through both classical synaptic transmission and volume transmission mechanisms that allow for widespread influence on neural networks involved in social cognition and emotional regulation. Oxytocin-producing neurons in the hypothalamic paraventricular nucleus (PVN) and supraoptic nucleus (SON) send extensive projections throughout the brain, including dense innervation of the amygdala, nucleus accumbens, bed nucleus of the stria terminalis, and other limbic structures critical for processing social and emotional information. These oxytocinergic pathways can release oxytocin both at classical synapses and through volume transmission, where the peptide diffuses through extracellular space to reach distant oxytocin receptors. This dual release mechanism enables oxytocin to coordinate activity across multiple brain regions simultaneously, facilitating the complex neural network interactions required for sophisticated social behaviors and emotional responses.
The central actions of oxytocin demonstrate remarkable specificity and context-dependence that reflect the sophisticated organization of brain oxytocin systems and their integration with other neurotransmitter networks. In the amygdala, oxytocin can both enhance and suppress neural activity depending on the specific subnuclei involved and the social context of the stimulus, with research showing that oxytocin facilitates processing of positive social cues while potentially reducing sensitivity to threatening or ambiguous social signals. In reward-related brain regions such as the nucleus accumbens and ventral tegmental area, oxytocin interacts with dopaminergic pathways to modulate the motivational salience of social stimuli and promote approach behaviors toward potential social partners. These region-specific effects are further modulated by individual differences in oxytocin receptor expression, genetic polymorphisms affecting the oxytocin system, and early life experiences that can permanently alter the development and function of central oxytocin pathways, contributing to the substantial individual variation observed in oxytocin's behavioral effects across different populations and experimental contexts.
Social Bonding and Attachment Research
The role of oxytocin in social bonding and attachment represents one of the most extensively studied and research-relevant aspects of its biology, with research spanning from fundamental animal studies to laboratory investigations of social behavior mechanisms in experimental models. The foundational work in prairie voles by Thomas Insel and colleagues demonstrated that oxytocin is essential for the formation of monogamous pair bonds, with oxytocin receptor antagonists preventing pair bond formation and oxytocin administration facilitating bonding even in the absence of mating. These findings established oxytocin as a critical neurochemical mediator of social attachment and spawned decades of research investigating how this neuropeptide influences human social behavior. Human studies have consistently demonstrated that intranasal oxytocin administration can enhance trust in economic games, increase empathy and perspective-taking abilities, and promote prosocial behaviors including charitable giving and cooperation, though these effects are often moderated by individual differences in personality, attachment style, and social context.
The molecular mechanisms underlying oxytocin's effects on social bonding involve complex interactions between oxytocin signaling and reward pathways that create positive associations with social stimuli and motivate continued social engagement. Oxytocin release during positive social interactions activates dopaminergic neurons in the ventral tegmental area and promotes dopamine release in the nucleus accumbens, creating rewarding feelings that become associated with specific social partners or social contexts. This neurochemical coupling between oxytocin and reward systems helps explain how social bonds are formed and maintained over time, as repeated positive social experiences become increasingly rewarding and motivating through oxytocin-dopamine interactions. Additionally, oxytocin's effects on synaptic plasticity and long-term potentiation in brain regions involved in learning and memory help consolidate social memories and strengthen the neural representations of important social relationships, contributing to the persistence and specificity of social bonds across time.
Research investigations exploring oxytocin's mechanisms in social behavior have yielded insights into complex neurobiological systems underlying social cognition. Laboratory studies in experimental models demonstrate oxytocin's effects on social recognition, attention, and communication behaviors through well-characterized neural pathways. Research investigations indicate that social behavioral parameters show modulation in experimental systems, and long-term studies are needed to determine whether oxytocin can produce sustained improvements in social functioning. Recent research has emphasized the importance of combining oxytocin administration with behavioral interventions and psychosocial support, as the neuropeptide appears to enhance social learning and attention to social cues rather than directly producing social improvements, suggesting that research applications may involve investigating oxytocin's role in facilitating social learning mechanisms.
Stress Reduction and HPA Axis Modulation
The stress-reducing properties of oxytocin represent a fundamental aspect of its biological function that has profound implications for understanding resilience, coping mechanisms, and the physiological basis of social support effects on health outcomes. Oxytocin exerts powerful inhibitory effects on the hypothalamic-pituitary-adrenal (HPA) axis at multiple levels, beginning with direct suppression of corticotropin-releasing hormone (CRH) secretion from paraventricular nucleus neurons in the hypothalamus. This central inhibitory action is complemented by oxytocin's ability to suppress adrenocorticotropic hormone (ACTH) release from the anterior pituitary and directly inhibit cortisol secretion from the adrenal cortex, creating a comprehensive dampening of stress hormone responses that promotes physiological calm and emotional regulation. Research in laboratory models demonstrates that elevated oxytocin signaling corresponds with reduced cortisol responses to social stressors, enhanced recovery from stress exposure, and improved stress resilience across mammalian species, highlighting the neuropeptide's role as an evolutionarily conserved stress-buffering system.
The molecular mechanisms underlying oxytocin's anti-stress effects involve sophisticated interactions with multiple neurotransmitter systems and signaling pathways that coordinate physiological and behavioral responses to environmental challenges. Oxytocin enhances GABAergic inhibition in stress-responsive brain regions, reducing neural excitability and promoting calm, relaxed states that facilitate social engagement and prosocial behavior. The neuropeptide also modulates noradrenergic and serotonergic signaling in ways that promote emotional regulation and reduce anxiety-like behaviors, while simultaneously enhancing parasympathetic nervous system activity that supports rest, digestion, and recovery processes. These coordinated effects on multiple stress-responsive systems create a comprehensive physiological state that opposes the fight-or-flight response and instead promotes what researchers have termed "tend-and-befriend" behaviors characterized by social approach, caregiving, and cooperation rather than aggression or withdrawal.
Laboratory research has revealed that oxytocin's stress-reducing effects are particularly prominent in social contexts, where the neuropeptide facilitates the social buffering of stress responses and enhances the benefits of social support for psychological and physical health. Laboratory studies demonstrate that oxytocin administration can modulate stress hormone responses to social challenges, enhance affiliative behaviors during conspecific interactions, and improve social bonding parameters in controlled experimental settings. The phenomenon of social buffering, where the presence of supportive others reduces stress responses, appears to be mediated in part by oxytocin release triggered by positive social contact including physical touch, eye contact, and emotional intimacy. This research has important implications for understanding how social relationships influence health outcomes and suggests research applications for investigating oxytocin's role in social support mechanisms and stress-related behavioral responses in laboratory models.
Pain Modulation and Analgesic Research
The analgesic properties of oxytocin have emerged as a particularly promising area of research given the urgent need for non-opioid pain management strategies and the unique mechanisms through which oxytocin modulates nociceptive processing. Unlike traditional analgesics that primarily target single receptor systems, oxytocin exerts multifaceted effects on pain pathways that involve both spinal and supraspinal mechanisms, creating comprehensive analgesia without the tolerance, dependence, or respiratory depression risks associated with opioid medications. At the spinal level, oxytocin activates glutamatergic interneurons in laminae I-II of the dorsal horn, which subsequently recruit GABAergic interneurons that increase local inhibition and suppress the transmission of nociceptive signals from primary afferents to ascending pain pathways. This spinal mechanism is complemented by oxytocin's ability to inhibit TRPV1 (vanilloid receptor 1) channels that mediate inflammatory and neuropathic pain responses, providing targeted relief for specific types of pain conditions.
The supraspinal analgesic mechanisms of oxytocin involve modulation of descending pain control systems and direct effects on brain regions involved in pain perception and emotional responses to painful stimuli. Oxytocin activates neurons in the periaqueductal gray and rostral ventromedial medulla that send inhibitory projections to spinal pain processing circuits, enhancing endogenous analgesic systems that normally function to suppress pain during times of stress or intense social interaction. Additionally, oxytocin's effects on emotional processing centers including the amygdala and anterior cingulate cortex can reduce the affective component of pain, making painful stimuli less distressing and aversive even when sensory intensity remains unchanged. These emotional analgesic effects are particularly relevant for chronic pain conditions where psychological factors significantly influence pain severity and functional impairment, suggesting that oxytocin-based interventions might address both the sensory and emotional dimensions of persistent pain.
Research investigating oxytocin's analgesic mechanisms has demonstrated efficacy across multiple pain models in experimental systems, with particular promise for neuropathic pain, inflammatory pain, and stress-induced hyperalgesia. Animal studies show that intrathecal oxytocin administration can reverse tactile allodynia and thermal hyperalgesia in nerve injury models, while systemic oxytocin reduces inflammatory pain responses and prevents stress-induced pain sensitization. Animal studies demonstrate that oxytocin administration can modulate nociceptive responses and alter pain sensitivity through well-characterized spinal and supraspinal mechanisms, with efficacy varying based on stress status and individual neurochemical profiles. The unique pharmacological profile of oxytocin, which combines direct analgesic effects with stress reduction and anxiolytic properties, positions it as a valuable research tool for investigating multi-modal approaches to nociceptive processing that integrate neurochemical, physiological, and behavioral mechanisms rather than focusing solely on single-pathway interventions.
Cardiovascular Effects and Cardioprotection
The cardiovascular effects of oxytocin represent a rapidly expanding area of research that has revealed sophisticated cardioprotective mechanisms operating through both direct actions on cardiac tissue and indirect effects mediated through autonomic nervous system modulation. Oxytocin exerts direct negative inotropic and chronotropic effects on cardiac muscle, reducing heart rate and contractility in ways that can improve myocardial efficiency and reduce cardiac workload during stress or pathological conditions. These direct cardiac effects are mediated through oxytocin receptors expressed in cardiomyocytes and involve calcium signaling pathways that modulate excitation-contraction coupling and cellular metabolism. Research has demonstrated that oxytocin can activate cardioprotective signaling cascades including the PI3K/Akt pathway, ERK1/2 kinases, and STAT3 transcription factors that promote cardiomyocyte survival, reduce apoptosis, and enhance cellular resistance to ischemic injury.
The cardioprotective mechanisms of oxytocin extend to profound anti-inflammatory and antioxidant effects that can prevent or limit cardiac damage during ischemia-reperfusion injury, myocardial infarction, and other acute cardiac events. Oxytocin reduces the production of pro-inflammatory cytokines including TNF-α, IL-1β, and IL-6 while enhancing anti-inflammatory mediators such as IL-10, creating a cytokine environment that favors tissue repair and regeneration rather than ongoing inflammatory damage. The neuropeptide also enhances antioxidant enzyme activity and reduces oxidative stress markers in cardiac tissue, protecting against reactive oxygen species that contribute to cardiomyocyte death and cardiac dysfunction. Additionally, oxytocin demonstrates pro-angiogenic properties that can enhance blood vessel formation and improve coronary circulation, while also exhibiting anti-fibrotic effects that prevent excessive scar tissue formation and preserve cardiac function following injury.
Clinical and experimental research has revealed that oxytocin's cardiovascular benefits extend beyond acute cardioprotection to include long-term effects on cardiovascular health and disease prevention. Animal studies demonstrate that elevated oxytocin signaling corresponds with improved vascular function, reduced blood pressure, and enhanced cardiovascular protective mechanisms in experimental models. Animal models of myocardial infarction demonstrate that oxytocin administration can reduce infarct size, preserve left ventricular function, and improve survival outcomes through multiple protective mechanisms operating in parallel. The regenerative properties of oxytocin, including its ability to stimulate stem cell differentiation toward cardiomyocyte lineages and promote tissue repair processes, suggest potential applications in cardiac regenerative medicine and tissue engineering approaches. These cardiovascular research applications position oxytocin as a valuable tool for investigating protective mechanisms in cardiac tissue and understanding endogenous cardioprotective pathways in laboratory models of cardiovascular physiology.
Maternal Behavior and Reproductive Research
The classical reproductive functions of oxytocin in maternal behavior and parturition represent the foundational research areas that established this neuropeptide's biological significance and continue to provide important insights into the neurobiological basis of caregiving, attachment, and reproductive success. During labor and delivery, oxytocin released from the posterior pituitary stimulates powerful uterine contractions through activation of oxytocin receptors in myometrial smooth muscle cells, with receptor sensitivity increasing dramatically during late pregnancy due to rising estrogen levels that upregulate receptor expression. This positive feedback system intensifies contractions as labor progresses, with mechanical stimulation of the cervix and birth canal triggering additional oxytocin release in a process that continues until delivery is completed. The milk ejection reflex represents another classical oxytocin function, where infant suckling stimulates oxytocin release that causes contraction of myoepithelial cells surrounding mammary alveoli, forcing milk into the ductal system for effective nursing.
Beyond these peripheral reproductive functions, central oxytocin release during parturition and lactation plays crucial roles in establishing and maintaining the mother-infant bond that is essential for infant survival and development. Brain oxytocin levels increase dramatically during labor and remain elevated during the early postpartum period, coinciding with the critical time window for maternal bonding and the onset of maternal behaviors including nursing, grooming, protection, and infant retrieval. This central oxytocin release activates neural circuits in the medial preoptic area, ventral tegmental area, and nucleus accumbens that coordinate maternal motivation and caregiving behaviors while simultaneously suppressing anxiety and fear responses that might interfere with effective mothering. Research in animal models has demonstrated that disruption of oxytocin signaling during this critical period can impair maternal behavior and bonding, while oxytocin administration can facilitate maternal responses even in virgin females, highlighting the neuropeptide's fundamental role in reproductive behavior programming.
Contemporary research into oxytocin's reproductive functions has expanded to encompass broader questions about parental behavior, family dynamics, and the neurobiological basis of caregiving across diverse family structures and species. Studies have revealed that fathers also show increased oxytocin levels and enhanced neural sensitivity to infant cues, with greater paternal oxytocin associated with more sensitive and responsive caregiving behaviors. Cross-cultural research has demonstrated that oxytocin's effects on parental behavior are conserved across different societies and child-rearing practices, while also revealing cultural variations in how oxytocin responses are shaped by social norms and expectations around parenting roles. These findings have important implications for understanding family functioning, developing interventions for parenting difficulties, and supporting healthy child development through enhanced parent-child relationships. The research applications of oxytocin in reproductive and maternal behavior studies continue to provide valuable insights into fundamental questions about attachment, caregiving motivation, and the biological foundations of family relationships across human and non-human species.
Conclusion
Oxytocin stands as one of the most remarkable and scientifically significant neuropeptides in modern biology, representing a paradigmatic example of how evolutionary ancient molecular systems can orchestrate complex social behaviors and physiological responses essential for survival and reproductive success. From Sir Henry Dale's initial discovery in 1906 to Vincent du Vigneaud's Nobel Prize-winning synthesis and the contemporary explosion of social neuroscience research, oxytocin has consistently revealed new dimensions of biological complexity and research potential that continue to reshape our understanding of behavior, emotion, and social cognition. The neuropeptide's dual nature as both a peripheral hormone mediating classical reproductive functions and a central neurotransmitter controlling sophisticated social behaviors exemplifies the elegant economy of biological systems, where single molecules can serve multiple functions across different physiological contexts while maintaining remarkable specificity and regulatory precision.
The comprehensive body of research on oxytocin provides an exceptional foundation for its continued investigation as a research tool for studying social neuroscience, stress physiology, and cardiovascular health. Its well-characterized receptor signaling mechanisms, documented effects across multiple physiological systems, and established safety profile in research investigations make it invaluable for researchers exploring fundamental questions about social behavior mechanisms, stress physiology, pain modulation, and the neurobiological basis of affiliative behaviors across mammalian species. The neuropeptide's unique ability to integrate social, emotional, and physiological responses while demonstrating context-dependent effects offers unprecedented opportunities for advancing research in social behavior mechanisms, stress response systems, pain modulation pathways, and cardiovascular physiology. As investigational techniques continue to advance and our understanding of oxytocin biology deepens, this neuropeptide will undoubtedly remain at the forefront of translational neuroscience research, providing insights into fundamental questions about mammalian social organization, affiliative bonding, and the molecular mechanisms that regulate complex behavioral phenotypes across diverse environmental and social contexts.
References
- Dale, H.H. (1906). On some physiological actions of ergot. Journal of Physiology 34(3):163-206. [doi.org]
- du Vigneaud, V. et al. (1953). The synthesis of an octapeptide amide with the hormonal activity of oxytocin. Journal of the American Chemical Society 75(19):4879-4880. [doi.org]
- Tuppy, H. (1953). The amino-acid sequence in oxytocin. Biochimica et Biophysica Acta 11(3):449-450. [doi.org]
- Gimpl, G. et al. (2001). The oxytocin receptor system: structure, function, and regulation. Physiological Reviews 81(2):629-683. [doi.org]
- Kosfeld, M. et al. (2005). Oxytocin increases trust in humans. Nature 435(7042):673-676. [doi.org]
- Carter, C.S. et al. (2020). Oxytocin, vasopressin and sociality. Progress in Brain Research 252:143-164. [doi.org]
- Heinrichs, M. et al. (2003). Social support and oxytocin interact to suppress cortisol and subjective responses to psychosocial stress. Biological Psychiatry 54(12):1389-1398. [doi.org]
- Goodin, B.R. et al. (2015). Intranasal oxytocin administration is associated with enhanced endogenous pain inhibition and reduced negative mood states. Clinical Journal of Pain 31(9):757-767. [doi.org]
- Ondrejcakova, M. et al. (2010). Oxytocin exerts direct cardioprotective effects via a seven transmembrane receptor in the rat heart. Regulatory Peptides 161(1-3):129-136. [doi.org]
- Bakos, J. et al. (2013). Combining oxytocin and naltrexone reduces stress reactivity in women. Psychopharmacology 227(2):309-318. [doi.org]
- Kemp, A.H. et al. (2012). Oxytocin increases heart rate variability in humans at rest: implications for social approach-related motivation and capacity for social engagement. PLoS One 7(8):e44014. [doi.org]
- Churchland, P.S. et al. (2010). Oxytocin: ethical issues. American Journal of Bioethics 10(1):15-17. [doi.org]
- Striepens, N. et al. (2011). Prosocial effects of oxytocin and clinical evidence for its therapeutic potential. Frontiers in Neuroendocrinology 32(4):426-450. [doi.org]
- Young, L.J. et al. (2011). The neurobiology of pair bonding. Nature Neuroscience 14(9):1048-1054. [doi.org]
- MacDonald, K. et al. (2011). A review of safety, side-effects and subjective reactions to intranasal oxytocin in human research. Psychoneuroendocrinology 36(8):1114-1126. [doi.org]
- Bethlehem, R.A.I. et al. (2013). Oxytocin, brain physiology, and functional connectivity: a review of intranasal oxytocin fMRI studies. Psychoneuroendocrinology 38(7):962-974. [doi.org]
- Alvares, G.A. et al. (2017). The oxytocin receptor gene (OXTR) in psychiatric and neurodevelopmental disorders: a systematic review and meta-analysis. Neuroscience and Biobehavioral Reviews 83:85-95. [doi.org]
- Shamay-Tsoory, S.G. et al. (2021). Intranasal administration of oxytocin increases envy and schadenfreude (gloating). Biological Psychiatry 89(4):315-322. [doi.org]
- Zhang, R. et al. (2020). Neuropeptide oxytocin mediates a calming effect on postoperative pain via the endogenous analgesia mechanism. Peptides 124:170-179. [doi.org]
- Yamasue, H. et al. (2020). Effect of intranasal oxytocin on the core social symptoms of autism spectrum disorder: a randomized clinical trial. Molecular Psychiatry 25(8):1849-1858. [doi.org]
- Sikich, L. et al. (2021). Intranasal oxytocin in children and adolescents with autism spectrum disorder. New England Journal of Medicine 385(16):1462-1473. [doi.org]
- Parker, K.J. et al. (2017). Intranasal oxytocin administration for social deficits and biomarkers of response in children with autism. Proceedings of the National Academy of Sciences 114(30):8119-8124. [doi.org]
| CAS Number | 50-56-6 |
|---|---|
| Molecular Formula | C43H66N12O12S2 |
| Molecular Weight | 1007.2 g/Mol |
| Sequence | H-DL-Cys(1)-DL-Tyr-DL-xiIle-DL-Gln-DL-Asn-DL-Cys(1)-DL-Pro-DL-Leu-Gly-NH2 |
Cosmic Peptides
Certificate of Analysis
Unable to load certificate. Download COA





