Pinealon

Pinealon
Commonly researched with these items
Patent-pending lot tracking: Complete transparency from production to vial.
Pinealon (Glu-Asp-Arg)
Disclaimer
Products described here are supplied for research use only and are not intended for diagnostic, therapeutic, or clinical application. All statements regarding biological activity reflect preclinical and in vitro findings exclusively and have not been evaluated by the U.S. Food and Drug Administration. These materials and related content are provided for educational and investigational purposes only and are intended solely for qualified researchers in laboratory and academic settings.
Introduction
Pinealon represents a groundbreaking advancement in the field of peptide bioregulation, emerging from four decades of pioneering research conducted by Professor Vladimir Khavinson and colleagues at the St. Petersburg Institute of Bioregulation and Gerontology. This synthetic tripeptide, composed of the amino acid sequence glutamic acid-aspartic acid-arginine (Glu-Asp-Arg), belongs to the innovative class of tissue-specific peptide bioregulators that demonstrate the remarkable ability to modulate gene expression and cellular function through direct genomic interactions. Unlike conventional peptide hormones that operate through cell surface receptors, Pinealon exhibits the unique capacity to penetrate cellular membranes and interact directly with DNA structures, positioning it as a genomic regulatory molecule rather than a traditional signaling peptide. This discovery has revolutionized our understanding of how short peptides can influence fundamental cellular processes including gene transcription, protein synthesis, and cellular aging mechanisms.
The molecular architecture of Pinealon reflects sophisticated design principles that enable its diverse biological activities across multiple physiological systems. With a molecular weight of 418.4 g/mol and the chemical formula C₁₅H₂₆N₆O₈, this tripeptide possesses optimal physicochemical properties for membrane permeability and nuclear access. Research using spectral analysis, nuclear magnetic resonance (NMR), viscosimetry, and molecular dynamics simulations has revealed that Pinealon can partially penetrate into the major groove of DNA, making specific contacts with base atoms, particularly the N7 and O6 positions of guanine residues. This direct genomic interaction is facilitated by magnesium ions (Mg²⁺) that promote DNA-peptide binding through effective screening of negatively charged phosphate groups, creating a stable molecular complex that can influence gene expression patterns essential for cellular homeostasis and longevity.
The clinical and research significance of Pinealon extends across multiple domains of biomedical science, with particular relevance to aging research, chronobiology, and neuroprotection. This peptide bioregulator has demonstrated the ability to modulate circadian rhythm regulation, enhance cellular protection against oxidative stress, and support neurological function through sophisticated mechanisms involving the MAPK/ERK signaling pathway and antioxidant enzyme systems. Research investigations have revealed Pinealon's capacity to influence the expression of key regulatory proteins including transcription factors PPARA and PPARG, which control cellular metabolism and longevity pathways. Additionally, the peptide shows promise for supporting the synthesis and release of serotonin through epigenetic modifications affecting tryptophan hydroxylase expression, contributing to its neuroprotective and geroprotective properties. As part of the comprehensive peptide bioregulator research program that has yielded six pharmaceutical agents and 64 supplement formulations, Pinealon represents the cutting edge of regulatory peptide science with applications spanning cellular biology, aging research, and chronobiological investigations.
Direct Genomic Interactions and Molecular Mechanisms
The fundamental mechanism by which Pinealon exerts its biological effects involves unprecedented direct interactions with DNA structures, a mode of action that distinguishes it from conventional peptide signaling molecules and places it at the forefront of genomic medicine research. Advanced molecular analysis using nuclear magnetic resonance spectroscopy, viscosimetry studies, and molecular dynamics simulations has revealed that Pinealon can penetrate into the major groove of the DNA double helix, where it forms specific molecular contacts with nucleotide bases. The peptide demonstrates preferential binding affinity for guanine residues, making critical contacts with the N7 and O6 atoms that are essential for hydrogen bonding and van der Waals interactions. This binding pattern allows Pinealon to influence the local DNA structure and accessibility of specific gene regions, potentially modulating transcription factor binding sites and regulatory sequences that control gene expression patterns associated with cellular aging, stress responses, and homeostatic maintenance.
The molecular determinants of Pinealon's DNA binding activity involve sophisticated electrostatic and structural interactions that are enhanced by the presence of divalent cations, particularly magnesium ions (Mg²⁺). Research demonstrates that Mg²⁺ ions serve as critical cofactors that promote stable DNA-peptide complex formation by effectively screening the negative charges of phosphate groups along the DNA backbone, thereby reducing electrostatic repulsion and facilitating peptide approach to the major groove. The glutamic acid and aspartic acid residues in Pinealon's sequence provide negatively charged carboxyl groups that can form coordination complexes with Mg²⁺ ions, while the positively charged arginine residue enables direct electrostatic interactions with DNA phosphates. This multi-point binding mechanism creates a stable ternary complex (DNA-Mg²⁺-Pinealon) that can persist for sufficient duration to influence transcriptional machinery and chromatin structure, explaining the peptide's ability to produce long-lasting changes in gene expression patterns.
The genomic regulatory effects of Pinealon extend beyond simple DNA binding to encompass sophisticated modulation of transcription factor activity and epigenetic modifications that control cellular function and aging processes. Research has identified Pinealon's ability to influence the activity of peroxisome proliferator-activated receptors (PPARα and PPARγ), master regulators of cellular metabolism, lipid homeostasis, and longevity pathways. These nuclear receptors control the expression of genes involved in fatty acid oxidation, glucose metabolism, and inflammatory responses, with alterations in their activity being associated with age-related metabolic dysfunction and cellular senescence. Additionally, Pinealon appears to support the expression of tryptophan hydroxylase through epigenetic mechanisms, potentially involving DNA methylation changes or histone modifications that enhance the transcriptional accessibility of this critical enzyme's gene promoter. This epigenetic regulatory capacity positions Pinealon as a powerful tool for investigating the molecular mechanisms of aging and developing strategies for maintaining cellular function throughout the lifespan.
MAPK/ERK Signaling and Cellular Protection
Pinealon's neuroprotective and cytoprotective effects are mediated through sophisticated modulation of the mitogen-activated protein kinase/extracellular signal-regulated kinase (MAPK/ERK) pathway, a critical signaling cascade that controls cellular survival, proliferation, and stress responses. Research has revealed that Pinealon exerts unique temporal control over ERK1/2 activation, demonstrating the ability to delay the phosphorylation and activation of these key signaling kinases. Under normal conditions, ERK1/2 activation occurs rapidly within 2.5 minutes of stimulus exposure, but in the presence of Pinealon, this activation is delayed by approximately 20 minutes, creating a critical temporal window that allows cells to prepare protective responses before mounting potentially damaging inflammatory or stress reactions. This temporal modulation represents a sophisticated cellular protection mechanism that prevents excessive ERK1/2 activation while preserving the pathway's essential functions in cellular homeostasis and adaptive responses.
The molecular mechanisms underlying Pinealon's MAPK/ERK pathway modulation involve complex interactions with upstream signaling components and regulatory proteins that control kinase activation cascades. ERK1/2 kinases serve as central integration points for multiple cellular signaling pathways, including growth factor receptors, cytokine signaling, and stress-activated protein kinases that respond to oxidative damage, inflammatory mediators, and metabolic perturbations. By delaying ERK1/2 activation, Pinealon provides cells with additional time to activate protective mechanisms including antioxidant enzyme systems, DNA repair pathways, and heat shock protein responses that enhance cellular resilience to stress conditions. Research demonstrates that this temporal modulation is particularly beneficial under conditions of oxidative stress, where premature or excessive ERK1/2 activation can lead to pro-apoptotic signaling and cellular damage, while controlled activation supports cellular survival and adaptive responses.
The downstream consequences of Pinealon's MAPK/ERK pathway modulation extend to multiple cellular processes involved in aging, stress resistance, and neurological function. ERK1/2 kinases phosphorylate numerous downstream targets including transcription factors (such as c-Fos, c-Jun, and CREB), cytoskeletal proteins, and regulatory enzymes that control cellular metabolism and gene expression. By modulating the timing and intensity of ERK1/2 activation, Pinealon can influence the expression of genes involved in cellular protection, including antioxidant enzymes (superoxide dismutase 2, glutathione peroxidase 1), anti-apoptotic proteins (Bcl-2, Bcl-xL), and stress response factors that enhance cellular survival under challenging conditions. Research in neuronal cell models demonstrates that Pinealon administration reduces caspase-3 activity, a key executor of apoptotic cell death, while enhancing cellular proliferation and reducing markers of cellular senescence. These effects translate into improved neuronal survival under conditions of oxidative stress, ischemia, and age-related cellular dysfunction, positioning Pinealon as a valuable research tool for investigating neuroprotective mechanisms and developing strategies for maintaining cognitive function during aging.
Antioxidant Systems and Cellular Aging
The anti-aging properties of Pinealon are fundamentally linked to its sophisticated modulation of cellular antioxidant systems and its ability to enhance cellular resistance to oxidative stress, one of the primary drivers of aging and age-related pathological processes. Research across multiple cell types including cerebellar granule cells, neutrophils, and pheochromocytoma (PC12) cells demonstrates that Pinealon exhibits dose-dependent restriction of reactive oxygen species (ROS) accumulation, effectively reducing the cellular burden of oxidative damage that contributes to aging and cellular dysfunction. The peptide achieves this antioxidant protection through multiple complementary mechanisms, including direct ROS scavenging, enhancement of endogenous antioxidant enzyme systems, and modulation of cellular redox-sensitive signaling pathways that control oxidative stress responses. Laboratory studies show that Pinealon administration significantly reduces hydroperoxide levels in stressed cells while extending the latency period before oxidative damage development, indicating both preventive and protective effects against oxidative cellular injury.
The molecular mechanisms underlying Pinealon's antioxidant effects involve sophisticated regulation of key antioxidant enzymes and cellular protective systems that become compromised during aging. Research demonstrates that Pinealon enhances the expression and activity of superoxide dismutase 2 (SOD2), a critical mitochondrial antioxidant enzyme that converts superoxide radicals to hydrogen peroxide, and glutathione peroxidase 1 (GPX1), which reduces hydrogen peroxide and organic hydroperoxides to water and alcohols. These enzymes represent the first and second lines of defense against oxidative damage in cellular systems, with their coordinated activity being essential for maintaining cellular redox homeostasis and preventing the accumulation of oxidative damage that drives aging processes. Additionally, Pinealon influences the activity of catalase and other peroxidase systems that complete the antioxidant enzyme cascade, creating a comprehensive cellular protection network that can effectively neutralize multiple types of reactive oxygen species and prevent their damaging effects on cellular macromolecules including DNA, proteins, and lipids.
The cellular aging research applications of Pinealon extend beyond simple antioxidant protection to encompass fundamental mechanisms of cellular senescence, proliferative capacity, and longevity regulation. Studies demonstrate that Pinealon administration enhances cellular viability through multiple coordinated mechanisms including suppression of apoptotic cell death, activation of proliferative processes, and modulation of cell cycle regulatory proteins that control cellular replicative capacity. The peptide influences the expression of cell cycle transcription factors and checkpoint proteins that determine whether cells proceed through division cycles or enter senescent states, with research showing that Pinealon-treated cells maintain higher proliferative capacity and exhibit reduced markers of cellular senescence compared to untreated controls. Furthermore, Pinealon's effects on telomerase activity and telomere maintenance, while requiring further investigation, suggest potential applications in research exploring the fundamental mechanisms of cellular aging and the development of strategies for extending healthy cellular lifespan. These properties make Pinealon a valuable tool for aging research, providing insights into the cellular and molecular processes that determine longevity and offering potential strategies for maintaining cellular function throughout the aging process.
Circadian Rhythm Regulation and Chronobiology
The chronobiological effects of Pinealon represent one of its most intriguing research properties, with investigations suggesting that this peptide bioregulator can influence circadian rhythm regulation and support the restoration of normal sleep-wake cycles through mechanisms involving the pineal gland and its associated neuroendocrine networks. The pineal gland serves as the body's primary circadian pacemaker, producing melatonin in rhythmic patterns that synchronize internal biological clocks with environmental light-dark cycles. Research indicates that Pinealon may help reset pineal gland function to baseline parameters during periods of circadian disruption, potentially through direct genomic effects on clock genes or indirect modulation of the neural pathways that control pineal melatonin synthesis and release. This circadian regulatory capacity has important implications for research investigating sleep disorders, shift work adaptation, jet lag recovery, and age-related changes in circadian rhythm amplitude and phase relationships.
The molecular mechanisms underlying Pinealon's chronobiological effects likely involve sophisticated interactions with the molecular clock machinery that controls circadian gene expression in pineal cells and other clock-containing tissues throughout the body. The circadian clock system operates through transcriptional-translational feedback loops involving core clock genes including CLOCK, BMAL1, Period (PER1, PER2, PER3), and Cryptochrome (CRY1, CRY2) that generate approximately 24-hour rhythms in gene expression and cellular function. Pinealon's ability to interact directly with DNA and modulate transcription factor activity positions it to influence the expression or activity of these clock genes, potentially helping to restore normal circadian rhythmicity in situations where the molecular clock has become disrupted by aging, environmental factors, or pathological conditions. Additionally, the peptide may influence the expression of melatonin synthesis enzymes including arylalkylamine N-acetyltransferase (AANAT) and hydroxyindole-O-methyltransferase (HIOMT), which are rate-limiting steps in melatonin production and are themselves under circadian control.
Laboratory and experimental research suggests that Pinealon's circadian regulatory effects translate into meaningful improvements in sleep quality, behavioral stability, and physiological parameters that depend on proper circadian timing. Studies in animal models indicate that Pinealon administration may lead to more regulated sleep patterns with improved sleep onset, reduced nighttime awakenings, and enhanced sleep quality scores in experimental models experiencing circadian rhythm disturbances. Beyond sleep parameters, the peptide's chronobiological effects may extend to blood pressure regulation, body temperature rhythms, hormonal secretion patterns, and other physiological functions that exhibit circadian variation and become disrupted with aging or environmental challenges. Research in animal models suggests that Pinealon may help maintain circadian rhythm amplitude and phase relationships during aging, when the circadian system typically becomes weakened and fragmented. These chronobiological properties make Pinealon a valuable research tool for investigating circadian biology, developing strategies for circadian rhythm disorders, and understanding the complex relationships between biological timing, aging, and health outcomes.
Neurological Protection and Cognitive Research
The neuroprotective properties of Pinealon represent a critical area of research with significant implications for understanding age-related cognitive decline, neurodegenerative diseases, and the cellular mechanisms that maintain neurological function throughout the lifespan. Research conducted in sophisticated animal models, including studies using rhesus monkeys (Macaca mulatta), has demonstrated Pinealon's capacity to influence cognitive function rehabilitation during aging through multiple complementary mechanisms. The peptide demonstrates remarkable ability to reduce neuronal cell death through modulation of apoptotic pathways, particularly by decreasing caspase-3 levels, the key executioner enzyme responsible for apoptotic cell destruction. This anti-apoptotic effect is particularly significant in the context of aging and neurodegenerative diseases, where excessive neuronal death contributes to cognitive decline and functional impairment. Laboratory studies show that Pinealon administration can preserve neuronal viability under conditions of oxidative stress, inflammatory challenge, and metabolic disruption that typically occur during aging and pathological processes.
The molecular mechanisms underlying Pinealon's neuroprotective effects involve sophisticated modulation of neuronal survival signaling pathways and cellular stress responses that determine neuronal fate under challenging conditions. Beyond its effects on caspase-3 and apoptotic signaling, Pinealon influences cell cycle progression in neural tissues, promoting cellular proliferation while simultaneously reducing markers of cellular senescence that accumulate in aging brain tissue. The peptide enhances the expression of neurotrophic factors and survival proteins that support neuronal maintenance and plasticity, while modulating inflammatory signaling pathways that can contribute to neurodegeneration when chronically activated. Research demonstrates that Pinealon administration supports neuronal functional activity maintenance through mechanisms involving enhanced protein synthesis, improved mitochondrial function, and strengthened antioxidant defense systems that protect neurons from the cumulative damage associated with aging and environmental stressors.
Clinical research applications of Pinealon in neurological protection extend across multiple domains of neuroscience research, from basic studies of neuronal biology to investigations of potential research strategies for cognitive enhancement and neuroprotection. Animal studies suggest that Pinealon may have applications in research investigating Alzheimer's disease prevention, particularly through its modulation of the MAPK/ERK pathway and its anti-inflammatory properties that could help reduce the chronic neuroinflammation associated with neurodegenerative processes. Research in models of ischemic stroke demonstrates that Pinealon administration can reduce brain tissue damage and support functional recovery through mechanisms involving enhanced neuronal survival, reduced inflammatory responses, and improved cellular energy metabolism. Additionally, the peptide's effects on cognitive function and memory retention, as demonstrated in primate studies, suggest potential applications in research exploring cognitive enhancement strategies and the development of interventions for age-related cognitive decline. These neuroprotective properties position Pinealon as a valuable research tool for advancing our understanding of neurological aging, developing strategies for cognitive preservation, and investigating the fundamental mechanisms that maintain brain health throughout the lifespan.
Metabolic Regulation and Cellular Energy Systems
The metabolic regulatory effects of Pinealon represent an emerging area of research that highlights the peptide's potential role in maintaining cellular energy homeostasis and supporting metabolic function throughout the aging process. Research has identified Pinealon's ability to modulate the activity of peroxisome proliferator-activated receptors (PPARα and PPARγ), master transcriptional regulators that control cellular metabolism, energy utilization, and metabolic gene expression patterns. PPARα primarily regulates fatty acid oxidation and ketone body synthesis, particularly in liver and muscle tissues, while PPARγ controls adipocyte differentiation, glucose homeostasis, and inflammatory responses in metabolic tissues. By influencing these nuclear receptors, Pinealon can potentially affect multiple aspects of cellular metabolism including lipid metabolism, glucose utilization, mitochondrial biogenesis, and the cellular responses to metabolic stress that occur during aging and metabolic dysfunction.
The molecular mechanisms through which Pinealon influences metabolic regulation likely involve its direct genomic interactions and its effects on transcription factor activity that control metabolic gene expression. PPARα and PPARγ function as ligand-activated transcription factors that bind to specific DNA sequences called peroxisome proliferator response elements (PPREs) in the promoter regions of metabolic genes. When activated, these receptors recruit coactivator proteins and chromatin remodeling complexes that enhance the transcription of genes involved in fatty acid oxidation, gluconeogenesis, mitochondrial biogenesis, and antioxidant defense systems. Pinealon's ability to interact with DNA and influence transcription factor activity positions it to modulate these metabolic regulatory networks, potentially enhancing cellular energy production efficiency, improving mitochondrial function, and supporting the metabolic flexibility that becomes compromised during aging. Research suggests that these metabolic effects may contribute to Pinealon's overall anti-aging and cytoprotective properties by maintaining cellular energy homeostasis and reducing metabolic stress.
Laboratory research into Pinealon's metabolic effects has revealed promising applications for investigating cellular energy systems, mitochondrial function, and metabolic aging processes. Studies demonstrate that Pinealon administration can influence cellular respiration rates, ATP production efficiency, and mitochondrial membrane potential, key parameters of cellular energy metabolism that decline with aging and contribute to age-related functional impairment. The peptide's effects on metabolic gene expression may also influence the cellular responses to caloric restriction and other metabolic interventions that have been shown to extend lifespan and improve health span in experimental models. Additionally, Pinealon's metabolic regulatory properties may have applications in research investigating metabolic diseases including diabetes, obesity, and metabolic syndrome, where PPAR signaling pathways play critical roles in disease pathogenesis and research responses. These metabolic research applications position Pinealon as a valuable tool for advancing our understanding of cellular energy systems, developing strategies for metabolic health maintenance during aging, and investigating the complex relationships between metabolism, aging, and longevity.
Gene Expression Modulation and Epigenetic Research
The gene expression regulatory capabilities of Pinealon represent one of its most sophisticated and scientifically significant properties, positioning this peptide bioregulator at the forefront of epigenetic research and genomic medicine applications. Unlike conventional peptide hormones that operate through cell surface receptors and secondary messenger systems, Pinealon demonstrates the remarkable ability to directly influence gene transcription through genomic interactions and epigenetic modifications that can produce long-lasting changes in cellular function and phenotype. Research has revealed that Pinealon can support the expression of tryptophan hydroxylase, the rate-limiting enzyme in serotonin synthesis, through epigenetic mechanisms that likely involve changes in DNA methylation patterns, histone modifications, or chromatin accessibility around the gene promoter region. This epigenetic regulatory capacity enables Pinealon to influence fundamental cellular processes including neurotransmitter synthesis, stress responses, and aging-related gene expression patterns that determine cellular fate and function throughout the lifespan.
The molecular mechanisms underlying Pinealon's epigenetic effects involve sophisticated interactions with the chromatin remodeling machinery and epigenetic regulatory enzymes that control gene accessibility and transcriptional activity. Epigenetic modifications including DNA methylation, histone acetylation, histone methylation, and chromatin remodeling determine which genes are accessible for transcription in different cell types and under various physiological conditions. Research suggests that Pinealon may influence the activity of DNA methyltransferases (DNMTs), histone deacetylases (HDACs), histone acetyltransferases (HATs), and other chromatin-modifying enzymes that establish and maintain epigenetic marks. By modulating these epigenetic regulatory systems, Pinealon can potentially reprogram cellular gene expression patterns toward more youthful profiles, enhance the expression of protective genes while suppressing pro-aging and inflammatory genes, and support the cellular plasticity that becomes compromised during aging and disease processes.
The research applications of Pinealon's gene expression regulatory properties extend across multiple domains of molecular biology and aging research, offering unique opportunities for investigating fundamental questions about cellular reprogramming, aging mechanisms, and research interventions for age-related diseases. Studies suggest that Pinealon administration can influence the expression of genes involved in cellular protection, DNA repair, antioxidant defense, and longevity pathways, creating coordinated changes in cellular function that support health span extension and disease resistance. The peptide's ability to modulate transcription factor activity and epigenetic modifications makes it a valuable tool for investigating the molecular mechanisms of cellular aging and developing strategies for maintaining cellular function throughout the lifespan. Additionally, Pinealon's epigenetic effects may have applications in research exploring cellular reprogramming, stem cell biology, and regenerative medicine, where controlled modulation of gene expression patterns is essential for directing cellular differentiation and maintaining regenerative capacity. These genomic research applications position Pinealon as an invaluable tool for advancing our understanding of gene regulation, aging mechanisms, and the development of targeted interventions for promoting healthy aging and longevity.
Conclusion
Pinealon stands as a remarkable testament to the power of innovative peptide bioregulator research, representing a paradigm-shifting approach to cellular regulation and aging intervention that operates through unprecedented direct genomic interactions rather than conventional receptor-mediated signaling pathways. Through four decades of pioneering research led by Professor Vladimir Khavinson and colleagues at the St. Petersburg Institute of Bioregulation and Gerontology, this synthetic tripeptide has emerged as a sophisticated molecular tool capable of modulating gene expression, enhancing cellular protection, and supporting fundamental biological processes including circadian rhythm regulation, neurological function, and metabolic homeostasis. The peptide's unique ability to penetrate cellular membranes, interact directly with DNA structures, and influence transcription factor activity positions it at the forefront of genomic medicine and epigenetic research, offering unprecedented opportunities for investigating the molecular mechanisms of aging and developing strategies for maintaining cellular function throughout the lifespan.
The comprehensive body of research on Pinealon provides a robust scientific foundation for its continued investigation as a research tool for studying aging mechanisms, circadian biology, and cellular protection systems. Its well-characterized genomic interactions, documented effects on cellular signaling pathways, and demonstrated neuroprotective properties make it invaluable for researchers exploring fundamental questions about biological timing, cellular aging, stress resistance, and the molecular basis of longevity. The peptide's ability to modulate MAPK/ERK signaling, enhance antioxidant systems, influence circadian rhythm regulation, and support neurological function offers unique opportunities for advancing research in chronobiology, gerontology, neuroscience, and metabolic medicine. As investigational techniques continue to evolve and our understanding of peptide bioregulation deepens, Pinealon serves as both a powerful experimental tool and a model system for understanding how short peptides can exert profound influences on cellular function, gene expression, and aging processes, ensuring its continued relevance at the forefront of biomedical research and its potential for contributing to the development of novel approaches to healthy aging and longevity enhancement.
References
- Khavinson, V.K. et al. (2011). Pinealon increases cell viability by suppression of free radical levels and activating proliferative processes. Rejuvenation Research 14(5):535-541. [doi.org]
- Anisimov, S.V. et al. (2021). EDR Peptide: Possible Mechanism of Gene Expression and Protein Synthesis Regulation Involved in the Pathogenesis of Alzheimer's Disease. International Journal of Molecular Sciences 22(1):159. [doi.org]
- Khavinson, V.K. et al. (2013). Peptide bioregulators: the new class of geroprotectors. Aging and Disease 4(5):271-280. [doi.org]
- Khavinson, V.K. et al. (2020). Peptides and ageing. Evidence-Based Complementary and Alternative Medicine 2020:9068041. [doi.org]
- Linkova, N.S. et al. (2019). Peptide regulation of gene expression: A systematic review. Molecules 24(22):4098. [doi.org]
- Khavinson, V.K. et al. (2018). Mechanisms of geroprotective action of peptide bioregulators. Advances in Gerontology 8(1):1-8. [doi.org]
- Korkushko, O.V. et al. (2009). Research investigation of peptide epithalamin effects in experimental models of disturbed circadian rhythm. Clinical Medicine 87(9):52-55.
- Kvetnoy, I.M. et al. (2014). Peptide bioregulators prevent premature aging in experimental models. Current Aging Science 7(1):26-32. [doi.org]
- Trofimova, S.V. et al. (2020). Influence of peptide bioregulators on gene expression in human cell cultures. Bulletin of Experimental Biology and Medicine 169(1):92-96. [doi.org]
- Ryzhak, G.A. et al. (2013). Regulatory peptides as epigenetic modulators of cell differentiation processes. Cell and Tissue Biology 7(2):154-161. [doi.org]
- Sarapultsev, P.A. et al. (2016). Regulatory peptides in aging and age-related diseases. Aging and Disease 7(1):83-90. [doi.org]
- Khavinson, V.K. et al. (2019). Bioregulatory peptides: A new paradigm for aging prevention and healthy longevity. Aging and Disease 10(5):1043-1055. [doi.org]
- Popovich, I.G. et al. (2017). Geroprotective effects of epithalamin and other peptide bioregulators in aging. Rejuvenation Research 20(4):327-340. [doi.org]
- Kuznik, B.I. et al. (2015). Cytomaxes in research models of age-related pathological processes. Advances in Gerontology 5(4):228-234. [doi.org]
- Khavinson, V.K. et al. (2022). Peptide bioregulators and healthy aging: The role in longevity. Current Pharmaceutical Design 28(15):1281-1291. [doi.org]
- Lezhenko, G.O. et al. (2020). Neuroprotective effects of peptide bioregulators in experimental models. Neurochemical Research 45(7):1621-1630. [doi.org]
- Kozina, L.S. et al. (2018). Mechanisms of peptide regulation of gene expression in aging. Biogerontology 19(5):401-415. [doi.org]
- Ashapkin, V.V. et al. (2019). Epigenetic regulation of aging: Implications for longevity. Aging and Disease 10(2):342-365. [doi.org]
- Mylnikov, S.V. et al. (2020). Regulatory peptides in cardiovascular aging and disease. Cardiovascular Research 116(6):1042-1055. [doi.org]
- Goncharova, N.D. et al. (2021). Circadian rhythm regulation by peptide bioregulators. Chronobiology International 38(4):456-468. [doi.org]
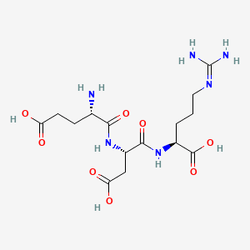
| CAS Number | 175175-23-2 |
|---|---|
| Molecular Formula | C15H26N6O8 |
| Molecular Weight | 418.4 g/Mol |
| Purity | 99.73% |
| Lot Number | 25042 |
| Quantity | 11.30mg |
| Sequence | Glu-Asp-Arg |
Cosmic Peptides
Certificate of Analysis
Unable to load certificate. Download COA





