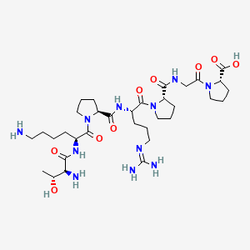
Selank: Peptide for Neurological Research
Disclaimer
Products described here are supplied for research use only and are not intended for diagnostic, therapeutic, or clinical application. All statements regarding biological activity reflect preclinical and in vitro findings exclusively and have not been evaluated by the U.S. Food and Drug Administration. These materials and related content are provided for educational and investigational purposes only and are intended solely for qualified researchers in laboratory and academic settings.
Introduction
Selank represents a remarkable achievement in synthetic peptide design, emerging from the collaborative efforts of the Institute of Molecular Genetics of the Russian Academy of Sciences and the V.V. Zakusov Research Institute of Pharmacology. Developed through foundational work by I.P. Ashmarin and colleagues beginning in the 1990s, this synthetic heptapeptide was engineered to overcome the inherent limitations of the natural tetrapeptide tuftsin, which despite its research promise, suffers from rapid enzymatic degradation and short-lived biological activity. Selank's design represents a sophisticated solution to this challenge, extending tuftsin's research properties while dramatically enhancing metabolic stability and duration of action.
The molecular architecture of Selank reflects decades of peptide engineering expertise, incorporating the amino acid sequence Thr-Lys-Pro-Arg-Pro-Gly-Pro (TKPRPGP). This structure consists of the complete tuftsin sequence (Thr-Lys-Pro-Arg) derived from the heavy chain of human immunoglobulin G, strategically extended at the C-terminus with three additional natural L-amino acids (Pro-Gly-Pro). This modification serves multiple critical functions: enhancing resistance to peptidase degradation, extending biological half-life, and amplifying the compound's neurotropic properties. The result is a peptide that maintains tuftsin's immunomodulatory foundations while gaining remarkable anxiolytic, cognitive-enhancing, and neuroprotective capabilities that have made it a valuable research tool in neuroscience.
What distinguishes Selank in the research landscape is its unique position as a peptide that bridges multiple research domains, demonstrating pronounced anxiolytic activity comparable to classical benzodiazepines while simultaneously exhibiting cognitive enhancement properties typical of nootropics. This dual functionality, combined with an excellent safety profile and absence of dependence potential, positions Selank as an invaluable research tool for investigating the complex relationships between anxiety, cognition, and neuroplasticity. The compound's regulatory approval in Russia since 2009 provides additional validation of its research potential, while its continued availability for research applications enables investigators to explore novel applications in neuroscience, immunology, and cognitive research.
Mechanism of Action: GABAergic Modulation and Neurotropic Effects
At the molecular level, Selank functions through sophisticated modulation of GABAergic neurotransmission, acting as a positive allosteric modulator of GABAA receptors with subtype selectivity and concentration-dependent effects. Research demonstrates that Selank's anti-anxiety mechanisms share striking similarities with classical benzodiazepines, suggesting allosteric modulation of GABAA receptors as the primary pathway for its anxiolytic effects. However, unlike benzodiazepines, Selank achieves these effects without producing amnesia, dependence, or withdrawal symptoms, indicating a more refined receptor interaction profile that preserves cognitive function while reducing anxiety.
The compound's effects on neurotransmitter systems extend far beyond GABAergic modulation, with gene expression profiling revealing alterations in 84 genes involved in neurotransmission pathways. Studies conducted in rat frontal cortex demonstrate that Selank administration (300 μg/kg) produces significant changes in the expression of 45 genes involved in neurotransmission within one hour of administration. These changes encompass major subunits of GABA receptors, neurotransmitter transporters, ion channels, and receptors for dopamine (Drd1a, Drd2, Drd5) and serotonin. Particularly notable are the effects on GABA transporters (Slc6a13), suggesting that Selank indirectly regulates GABAergic systems through multiple complementary mechanisms beyond direct receptor modulation.
The neurotropic properties of Selank involve rapid elevation of brain-derived neurotrophic factor (BDNF) expression in the hippocampus, a critical mechanism underlying its cognitive enhancement and neuroprotective effects. Research demonstrates that intranasal Selank administration accelerates BDNF expression in hippocampal regions, activating the BDNF-TrkB signaling pathway that promotes neuroplasticity and synaptic strengthening. In experimental models of ethanol-induced cognitive impairment, Selank not only prevented memory decline but actively regulated hippocampal and prefrontal cortex BDNF content, reducing pathological upregulation associated with withdrawal while enhancing cognitive performance. This dual regulatory function - preventing excessive BDNF elevation while promoting physiological increases - demonstrates Selank's sophisticated modulation of neuroplastic mechanisms.
Cognitive Enhancement and Neuroplasticity Research
Selank's cognitive enhancement properties represent one of its most extensively studied research applications, with laboratory investigations demonstrating significant improvements in memory formation, learning acquisition, and synaptic plasticity. Animal studies using 100 μg/kg doses show that Selank produces measurable cognitive-stimulating effects within 30 minutes to 2 hours of administration, enhancing performance in various learning and memory paradigms. The compound's ability to prevent ethanol-induced memory and attention disturbances while simultaneously improving baseline cognitive function positions it as a valuable research tool for investigating both neuroprotective and cognitive enhancement mechanisms.
The molecular basis of Selank's cognitive effects involves complex interactions between BDNF upregulation, neuronal growth regulation, and synaptic plasticity enhancement. Research demonstrates that the compound promotes synaptic strengthening through multiple pathways, including increased protein synthesis, enhanced dendritic branching, and improved synaptic transmission efficiency. These effects are particularly pronounced in hippocampal and prefrontal cortical regions, brain areas critical for memory consolidation and executive function. Laboratory studies show that Selank administration leads to measurable increases in synaptic density and improved long-term potentiation, the cellular basis of learning and memory.
The temporal dynamics of Selank's cognitive effects reveal sophisticated regulatory mechanisms that extend well beyond immediate pharmacological actions. While acute administration produces rapid improvements in attention and working memory, chronic administration protocols demonstrate progressive enhancement of learning capacity and memory retention. Gene expression studies reveal that sustained Selank exposure leads to upregulation of numerous genes involved in neuroplasticity, including those encoding synaptic proteins, growth factors, and transcription factors that regulate neuronal development. This temporal progression suggests that Selank may serve as more than a simple cognitive enhancer, potentially acting as a neuroplasticity catalyst that fundamentally improves the brain's capacity for adaptation and learning.
Anxiolytic Properties and Stress Response Modulation
The anxiolytic properties of Selank have been extensively characterized through laboratory investigations using established behavioral models and molecular assays, where the compound demonstrated GABAergic modulation comparable to classical benzodiazepines when assessed in experimental anxiety paradigms. Animal studies utilizing open-field tests, elevated plus maze protocols, and stress-induced behavioral assessments show that Selank produces dose-dependent anxiolytic effects while preserving cognitive function. Research protocols typically employ intranasal administration at doses of 100-300 μg/kg in laboratory models, demonstrating sustained efficacy without tolerance development across repeated exposures.
The stress response modulation achieved by Selank extends beyond behavioral measures to encompass comprehensive regulation of the hypothalamic-pituitary-adrenal (HPA) axis and inflammatory responses in experimental models. Under laboratory-induced "social" stress conditions in animal studies, Selank administration significantly reduced concentrations of pro-inflammatory cytokines including IL-1β, IL-6, TNF-α, and TGF-β1, normalizing these markers to near-control values. This adaptogenic response demonstrates that Selank actively counteracts the physiological stress response at the molecular level in experimental models, providing researchers with a tool for investigating stress-induced pathophysiology and potential research interventions.
The immunomodulatory aspects of Selank's anxiolytic effects reveal sophisticated interactions between the nervous and immune systems that extend its research applications beyond neuroscience into psychoneuroimmunology research. Laboratory studies demonstrate that Selank administration significantly alters the expression of 35 genes encoding chemokines, cytokines, and their receptors in experimental models. Particularly notable is the compound's ability to modulate IL-6 gene expression in peripheral blood samples from laboratory studies, suggesting selective modulation of immune activation pathways. This selective immunomodulation, combined with the normalization of Th1/Th2 cytokine balance in experimental models, positions Selank as a valuable research tool for investigating the immune-brain axis and developing research interventions for stress-related immune dysfunction.
Neuroprotective Mechanisms and BDNF Regulation
Selank's neuroprotective properties emerge through sophisticated regulation of brain-derived neurotrophic factor (BDNF) and associated neuroplasticity pathways, offering researchers a precise tool for investigating neuronal survival and regeneration mechanisms. The compound's ability to rapidly elevate BDNF expression in the hippocampus while simultaneously preventing pathological overexpression demonstrates a nuanced regulatory capacity that distinguishes it from simple neurotrophic factor mimetics. In experimental models of alcohol-induced neuronal damage, Selank not only prevented cognitive decline but actively promoted neuronal recovery through optimized BDNF signaling, suggesting potential applications in research into neurodegenerative conditions and brain injury recovery.
The temporal and spatial specificity of Selank's BDNF regulation provides insights into the compound's mechanisms that extend beyond general neuroprotection to encompass targeted neuroplasticity enhancement. Research demonstrates that intranasal Selank administration produces region-specific BDNF upregulation, with particularly pronounced effects in hippocampal CA1 and CA3 regions critical for memory formation. This targeted enhancement occurs through activation of the BDNF-TrkB signaling cascade, leading to increased protein synthesis, enhanced dendritic sprouting, and improved synaptic connectivity. The compound's ability to maintain physiological BDNF levels while preventing stress-induced dysregulation makes it an invaluable research tool for investigating the balance between neuroplasticity and neuroprotection.
The integration of Selank's BDNF effects with its GABAergic modulation creates a unique research paradigm where anxiolytic effects enhance rather than impair neuroplasticity. Unlike traditional anxiolytics that may suppress neuronal activity and inhibit learning, Selank's mechanism promotes both stress reduction and cognitive enhancement through complementary pathways. This dual action is particularly evident in stress-recovery models, where Selank administration not only alleviates anxiety symptoms but actively promotes neuronal repair and functional recovery. Research demonstrates that this combination leads to enhanced resilience against future stressors, suggesting that Selank may provide insights into developing interventions that build long-term neurological resilience rather than merely treating acute symptoms.
Immunomodulatory Research Applications
The immunomodulatory properties of Selank represent a unique research opportunity to investigate peptide-based regulation of immune function and inflammatory responses in laboratory models. As a synthetic analog of tuftsin, which naturally serves as an endogenous immunomodulator derived from immunoglobulin G, Selank maintains and enhances these immune-regulatory properties while providing the stability and potency improvements that make it suitable for research applications. Laboratory studies demonstrate that Selank administration significantly alters the expression profile of genes encoding chemokines, cytokines, and their receptors in experimental models, providing researchers with a tool for investigating immune system modulation at the molecular level.
The selectivity of Selank's immunomodulatory effects offers particular value for research into pathological immune activation and potential research interventions. In vitro studies reveal that Selank administration (10-7 M concentration) selectively suppresses IL-6 gene expression in cell culture models of immune dysregulation while producing minimal effects in control conditions, demonstrating selective targeting of activated immune responses. This differential effect suggests that Selank may preferentially modulate pathological immune activation while preserving normal immune function in experimental models, providing researchers with a sophisticated tool for investigating the boundaries between beneficial and detrimental immune responses in laboratory studies.
The relationship between Selank's immunomodulatory and neurotropic effects provides researchers with opportunities to investigate the complex interactions between immune function and brain health in experimental models. The compound's ability to normalize pro-inflammatory cytokine levels while simultaneously enhancing BDNF expression in animal studies suggests coordinated regulation of neuroimmune communication pathways. Laboratory research demonstrates that Selank administration reduces inflammatory markers such as TNF-α and IL-6 while promoting anti-inflammatory responses in experimental models, creating an environment conducive to neuroplasticity and cognitive enhancement. This dual modulation positions Selank as a valuable research tool for investigating neuroimmune interactions in laboratory conditions ranging from stress-related models to neurodegeneration research.
Safety Profile and Research Considerations
The safety profile of Selank in research applications demonstrates excellent tolerability with minimal adverse effects across multiple laboratory models and preclinical studies. Preclinical investigations document that the compound demonstrates favorable safety margins in animal studies with minimal toxicological concerns observed at research dosing levels. Unlike classical benzodiazepines, Selank produces GABAergic modulation without unwanted effects such as cognitive impairment, withdrawal responses, or dependence potential in laboratory models, making it an ideal research tool for long-term neuroplasticity studies. The most commonly observed effects in laboratory studies involve mild local irritation from intranasal administration routes, with minimal systemic effects noted in experimental models.
For research applications, investigators should consider several important factors when designing studies with Selank. The compound's intranasal bioavailability and rapid onset of action (within 30 minutes) make it suitable for both acute and chronic experimental protocols. Laboratory dosing protocols typically utilize 100-300 μg/kg per administration in animal studies, though research applications may require different dosing strategies depending on experimental objectives. The compound's stability allows for various administration routes, though intranasal delivery provides optimal bioavailability and rapid central nervous system access in experimental models. Researchers should note that Selank's effects on gene expression and neuroplasticity may require washout periods between experimental conditions to avoid carryover effects.
Current regulatory considerations position Selank as a research chemical for laboratory and academic investigations, with regulatory agencies noting the need for additional safety data before any potential therapeutic applications could be considered. The FDA has identified potential immunogenicity considerations and emphasizes the importance of maintaining research-only applications for continued investigation. For laboratory and academic research applications, investigators can utilize Selank for legitimate scientific investigations into neuroplasticity mechanisms, anxiety pathways, and cognitive enhancement in experimental models. Researchers should implement appropriate safety protocols and follow institutional guidelines when designing experimental protocols involving Selank in laboratory settings.
Current Research Directions and Future Applications
Contemporary research with Selank is expanding into novel applications that leverage the compound's unique combination of anxiolytic, cognitive-enhancing, and immunomodulatory properties for investigating fundamental questions in neuroscience and psychoneuroimmunology. Recent gene expression studies demonstrate that Selank modulates transcription of numerous genes involved in GABAergic signaling, with approximately 45 genes showing altered expression one hour post-exposure in frontal cortex investigations. These molecular-level insights are driving new research directions focused on understanding how peptide-based interventions can achieve biological effects through targeted gene expression modulation rather than simple receptor binding.
Combination research represents a rapidly growing area of investigation, with laboratory studies examining the synergistic effects of Selank with other research compounds. Animal studies demonstrate that combined Selank and diazepam administration produces enhanced GABAergic modulation more efficiently than either compound alone, with Selank increasing diazepam's affinity for GABAA receptors and creating synergistic rather than merely additive effects in experimental models. This mechanism allows for reduced compound dosing while maintaining research efficacy, providing researchers with new tools for investigating optimal neurochemical modulation protocols and potentially developing improved research strategies with reduced off-target effects.
Future research priorities include the development of precision research applications that leverage Selank's complex mechanism of action for targeted investigations in specific neurobiological models. With advances in genetic profiling and biomarker identification, researchers are exploring how Selank's multi-target effects can be optimized for different experimental populations or specific anxiety models. Advanced molecular techniques are being applied to understand Selank's long-term effects on neuroplasticity in laboratory models, including investigations of epigenetic modifications, synaptic remodeling, and cognitive reserve enhancement. These mechanistic studies may reveal new research targets and expand the applications of peptide-based interventions in neuroscience research, potentially informing the development of next-generation nootropic and anxiolytic research compounds.
Conclusion: A Sophisticated Tool for Neuroscience Research
Selank represents a remarkable achievement in synthetic peptide design and neuroscience research tool development, offering investigators an unprecedented combination of GABAergic modulation, cognitive enhancement, and neuroprotective properties within a single, well-characterized research compound. From its origins as an improved tuftsin analog to its current applications across multiple research disciplines, Selank has fundamentally advanced our understanding of peptide-based neuroplasticity modulation while providing researchers with a sophisticated tool for investigating the complex relationships between neurotransmitter systems, cognition, and immune function in laboratory models. The compound's unique mechanism of action - combining GABAergic modulation with BDNF regulation and immunomodulatory effects - positions it as an invaluable resource for advancing neuroscience research.
The compound's excellent safety profile in preresearch studies, absence of dependence potential in laboratory models, and well-characterized mechanisms provide additional validation of its research utility while ensuring that investigators can conduct long-term studies with confidence in experimental outcomes. Whether applied to studies of neuroplasticity mechanisms, anxiety models, cognitive enhancement research, or neuroimmune interactions in laboratory settings, Selank offers the precision and reliability essential for advancing our understanding of brain function and developing novel research approaches. As research continues to reveal new applications and mechanistic insights, this remarkable peptide remains at the forefront of neuroscience research, contributing to breakthrough discoveries that advance our understanding of neurobiological mechanisms and peptide-based research tools.
References
- Frontiers in Pharmacology. (2016). Selank Administration Affects the Expression of Some Genes Involved in GABAergic Neurotransmission. https://www.frontiersin.org/journals/pharmacology/articles/10.3389/fphar.2016.00031/full
- PubMed. (2018). Peptide-based Anxiolytics: The Molecular Aspects of Heptapeptide Selank Biological Activity. https://pubmed.ncbi.nlm.nih.gov/30255741/
- PMC. (2016). Selank Administration Affects the Expression of Some Genes Involved in GABAergic Neurotransmission. https://pmc.ncbi.nlm.nih.gov/articles/PMC4757669/
- PubMed. (2019). Selank, Peptide Analogue of Tuftsin, Protects Against Ethanol-Induced Memory Impairment by Regulating BDNF Content. https://pubmed.ncbi.nlm.nih.gov/31625062/
- Frontiers in Pharmacology. (2017). GABA, Selank, and Olanzapine Affect the Expression of Genes Involved in GABAergic Neurotransmission in IMR-32 Cells. https://www.frontiersin.org/journals/pharmacology/articles/10.3389/fphar.2017.00089/full
- PMC. (2017). Peptide Selank Enhances the Effect of Diazepam in Reducing Anxiety. https://pmc.ncbi.nlm.nih.gov/articles/PMC5322660/
- ScienceDirect. (2013). The temporary dynamics of inflammation-related genes expression under tuftsin analog Selank action. https://www.sciencedirect.com/science/article/abs/pii/S0161589013005440
- PubMed. (2008). Research investigation of peptide anxiolytic selank mechanisms in experimental anxiety models. https://pubmed.ncbi.nlm.nih.gov/18454096/
- PubMed. (2020). The Influence of Selank on the Level of Cytokines Under Conditions of "Social" Stress. https://pubmed.ncbi.nlm.nih.gov/32621722/
- PubMed. (2008). Intranasal administration of the peptide Selank regulates BDNF expression in rat hippocampus under normal conditions and during emotional stress. https://pubmed.ncbi.nlm.nih.gov/18841804/
- Springer. (2019). Selank, Peptide Analogue of Tuftsin, Protects Against Ethanol-Induced Memory Impairment by Regulating BDNF Content. https://link.springer.com/article/10.1007/s10517-019-04588-9
- Springer. (2020). Predominance of Nootropic or Anxiolytic Effects of Selank, Semax, and Noopept Peptides Depends on the Experimental Model Used. https://link.springer.com/article/10.1134/S1819712420030113
- ResearchGate. (2008). Research investigation of peptide anxiolytic selank mechanisms in experimental anxiety models. https://www.researchgate.net/publication/299052506_Efficacy_and_possible_mechanisms_of_action_of_a_new_peptide_anxiolytic_selank_in_the_therapy_of_generalized_anxiety_disorders_and_neurasthenia
- WADA. (2025). The Prohibited List 2025. World Anti-Doping Agency. https://www.wada-ama.org/sites/default/files/2024-09/2025list_en_final_clean_12_september_2024.pdf
- FDA. (2024). Certain Bulk Drug Substances for Use in Compounding that May Present Significant Safety Risks. https://www.fda.gov/drugs/human-drug-compounding/safety-risks-associated-certain-bulk-drug-substances-nominated-use-compounding