Semax: Advanced Melanocortin-Derived Peptide for Neurological Research
Disclaimer
Products described here are supplied for research use only and are not intended for diagnostic, therapeutic, or clinical application. All statements regarding biological activity reflect preclinical and in vitro findings exclusively and have not been evaluated by the U.S. Food and Drug Administration. These materials and related content are provided for educational and investigational purposes only and are intended solely for qualified researchers in laboratory and academic settings.
Introduction: A Breakthrough in Melanocortin-Derived Research
Semax represents a remarkable achievement in synthetic peptide design and neurological research tool development, emerging from the distinguished laboratories of the Institute of Molecular Genetics of the Russian Academy of Sciences under the guidance of academicians I.P. Ashmarin and N.F. Myasoedov. This synthetic heptapeptide, first developed in the late 1960s as part of a broader Russian initiative in peptide research, was engineered as an analog of the adrenocorticotropic hormone (ACTH) fragment 4-10, specifically designed to preserve the neurotropic effects of native melanocortins while eliminating hormonal activity. The compound's development represented a sophisticated approach to creating stable, research-grade peptides suitable for investigating complex neurobiological mechanisms.
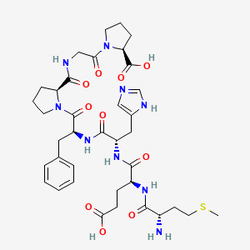
The molecular architecture of Semax reflects decades of peptide engineering expertise, incorporating the amino acid sequence Met-Glu-His-Phe-Pro-Gly-Pro (MEHFPGP). The name "Semax" derives from the Russian abbreviation for "seven amino acids," highlighting its heptapeptide structure. This design consists of the ACTH fragment (4-7) strategically extended with a C-terminal Pro-Gly-Pro tripeptide that ensures resistance to peptidase degradation while maintaining biological activity. The result is a peptide that functions as a synthetic melanocortin derivative with no hormonal activity but significant neurotropic properties, making it an invaluable tool for investigating melanocortin receptor systems and their roles in neurological processes.
What distinguishes Semax in the research landscape is its unique position as a melanocortin receptor antagonist that demonstrates pronounced neuroprotective and cognitive-enhancing properties in laboratory models. Unlike natural melanocortins that exhibit broad hormonal activities, Semax specifically targets neurobiological pathways while avoiding endocrine effects, positioning it as an ideal research tool for dissecting the specific contributions of melanocortin systems to brain function. The compound's regulatory status as a research chemical in most countries, combined with its extensive characterization in preclinical studies, enables investigators to explore novel applications in neuroscience research, neuroplasticity studies, and cognitive enhancement research while maintaining appropriate regulatory compliance.
Mechanism of Action: Melanocortin Receptor Modulation and BDNF Activation
At the molecular level, Semax functions as a competitive antagonist at melanocortin MC4 and MC5 receptors, demonstrating sophisticated selectivity for specific receptor subtypes within the melanocortin system. Laboratory investigations reveal that at 1 μM concentration, Semax effectively antagonizes the cAMP-inducing effects of α-melanocyte-stimulating hormone (α-MSH) in HEK293 cells expressing human MC4 receptors without affecting baseline cAMP levels. This selective antagonism distinguishes Semax from natural melanocortins and provides researchers with a precise tool for investigating melanocortin receptor function in neurobiological systems. The compound's ability to modulate these receptors without producing hormonal effects makes it particularly valuable for studies focused on the non-endocrine functions of melanocortin signaling.
The neurotropic effects of Semax involve sophisticated modulation of brain-derived neurotrophic factor (BDNF) and associated signaling pathways, with laboratory studies demonstrating a maximal 1.4-fold increase in BDNF protein levels accompanied by a 1.6-fold increase in TrkB tyrosine phosphorylation in rat hippocampus. Research shows that Semax administration produces a 3-fold increase in exon III BDNF mRNA expression, indicating transcriptional activation of BDNF synthesis rather than simple protein stabilization. These effects occur rapidly, with measurable changes in BDNF levels observed within 3 hours of intranasal administration at doses of 50-250 μg/kg in animal models. The temporal and magnitude specificity of these effects provides researchers with predictable and reproducible experimental conditions for investigating BDNF-dependent neuroplasticity mechanisms.
The neurotransmitter system interactions of Semax extend beyond BDNF modulation to encompass comprehensive activation of dopaminergic and serotonergic brain systems in laboratory models. Research demonstrates that Semax rapidly activates these neurotransmitter systems, showing positive modulatory effects on striatal neurotransmitter release in experimental settings. The compound's effects on neurotransmitter systems appear to be mediated through melanocortin receptor interactions rather than direct neurotransmitter binding, providing researchers with a tool for investigating the relationships between melanocortin signaling and classical neurotransmitter pathways. This mechanism offers unique research opportunities for understanding how peptide hormone systems integrate with monoaminergic neurotransmission in cognitive and behavioral regulation.
Neuroprotective Mechanisms and Gene Expression Modulation
Semax demonstrates sophisticated neuroprotective properties through comprehensive modulation of gene expression patterns related to inflammation, immune function, and neuronal survival in laboratory models of neurological injury. Research utilizing genome-wide transcriptional analysis in rat models of focal cerebral ischemia reveals that Semax administration significantly suppresses proinflammatory gene expression while enhancing the expression of genes related to immune system function and neuroprotection. These effects involve complex changes in mRNA expression patterns that compensate for disruptions caused by ischemia-reperfusion injury, providing researchers with insights into the molecular mechanisms underlying peptide-mediated neuroprotection.
The cerebroprotective effects of Semax extend to antioxidant mechanisms and metal-induced cytotoxicity prevention in experimental models. Laboratory investigations demonstrate that Semax possesses copper(II) binding capacity that prevents metal-induced cellular damage, suggesting additional mechanisms beyond receptor-mediated neuroprotection. In animal models of middle cerebral artery occlusion (MCAO), Semax administration (250 μg/kg for six daily administrations) resulted in reduced infarction size and improved performance on passive avoidance tasks compared to control groups. These findings position Semax as a valuable research tool for investigating multiple pathways of neuroprotection and their integration in preventing neurological damage.
The temporal dynamics of Semax's neuroprotective effects reveal region-specific activation patterns that provide researchers with insights into the spatial organization of peptide-mediated brain protection. Studies demonstrate that intranasal Semax administration at 50 and 250 μg/kg produces rapid increases in BDNF levels in rat basal forebrain but not cerebellum, indicating targeted regional effects that can be exploited for investigating brain region-specific vulnerabilities and protection mechanisms. The compound's ability to rapidly induce neurotrophin mRNAs in glial cell cultures further supports its utility as a research tool for investigating glial-neuronal interactions in neuroprotection and recovery processes.
Cognitive Enhancement Research in Laboratory Models
The cognitive enhancement properties of Semax have been extensively characterized in laboratory animal models using standardized behavioral paradigms and learning assessments. Research utilizing passive avoidance tests in rat models demonstrates that Semax administration (250 μg/kg for 6 days) produces significant improvements in conditioned learning performance compared to control groups. These cognitive effects appear rapidly, with measurable improvements observed within 15 minutes of intranasal administration in healthy laboratory animals. The consistency and reproducibility of these effects across multiple behavioral paradigms make Semax a valuable research tool for investigating the neurobiological basis of learning and memory formation.
In experimental models of stress-induced cognitive impairment, Semax demonstrates protective effects against memory deficits without affecting baseline anxiety levels in laboratory animals. Research using food-motivated maze tasks in white rats shows that Semax administration (0.1 mg/kg intraperitoneally) effectively attenuates cognitive impairment caused by acute restraint stress while preserving normal stress responses. This selective protection of cognitive function provides researchers with a tool for dissecting the relationships between stress, anxiety, and cognitive performance in experimental settings. The compound's ability to preserve learning capacity under stress conditions offers insights into potential mechanisms for cognitive resilience and adaptation.
The molecular basis of Semax's cognitive enhancement effects involves complex interactions between BDNF upregulation, neurotransmitter system activation, and synaptic plasticity mechanisms in laboratory models. Research demonstrates that the cognitive improvements observed with Semax administration correlate with increased BDNF expression and TrkB phosphorylation in hippocampal regions critical for memory formation. The compound's effects on dopaminergic and serotonergic systems may contribute to enhanced attention and information processing, while BDNF-mediated neuroplasticity supports long-term memory consolidation. This multi-pathway approach provides researchers with a comprehensive tool for investigating the integration of different neurobiological systems in cognitive function.
Neurotransmitter System Research Applications
Semax offers researchers unique opportunities to investigate dopaminergic and serotonergic system modulation through melanocortin receptor-mediated mechanisms in laboratory settings. Recent research in animal models suggests that Semax administration influences both serotonergic and dopaminergic neurotransmitter systems, with potential applications for studying non-motor and motor behavioral patterns in experimental paradigms. However, laboratory studies indicate that low doses of Semax did not produce motor improvements in animal models, suggesting that its primary effects target cognitive and emotional processing systems rather than motor control pathways. This selectivity provides researchers with a tool for investigating the differential roles of neurotransmitter systems in various behavioral domains.
The relationship between Semax's melanocortin receptor antagonism and its neurotransmitter effects provides insights into the complex interactions between peptide hormone systems and classical neurotransmission pathways. Laboratory investigations demonstrate that Semax's effects on dopaminergic and serotonergic systems occur downstream of melanocortin receptor interactions rather than through direct neurotransmitter receptor binding. This mechanism offers researchers opportunities to investigate how peptide signaling modulates neurotransmitter function and how these interactions contribute to behavioral and cognitive outcomes in experimental models.
The temporal and dose-dependent characteristics of Semax's neurotransmitter effects have been characterized in multiple laboratory models, providing researchers with predictable experimental conditions for investigating neurotransmitter system function. Research shows that the activation of dopaminergic and serotonergic systems occurs rapidly following Semax administration, with effects measurable within the first hour of administration in animal models. The consistency of these effects across different experimental paradigms makes Semax a reliable research tool for investigating neurotransmitter system interactions and their roles in cognitive and behavioral regulation.
Advanced Molecular Research Applications
Recent advances in molecular research have positioned Semax as a valuable tool for investigating novel research targets and mechanisms in experimental models of neurological conditions. Breakthrough 2025 research demonstrates that Semax peptide targets the μ opioid receptor gene Oprm1 to promote deubiquitination and functional recovery in laboratory models of spinal cord injury, revealing previously unknown molecular targets for peptide-mediated neuroprotection. This discovery opens new research directions for investigating the interactions between melanocortin-derived peptides and opioid receptor systems in neurological recovery processes.
Advanced genomic studies utilizing RNA-Seq analysis have provided researchers with comprehensive insights into Semax's effects on genome-wide transcriptional patterns in laboratory models of cerebral ischemia. These investigations reveal that Semax administration produces coordinated changes in gene expression profiles that compensate for ischemia-induced disruptions, with particular emphasis on suppressing inflammatory genes while activating neurotransmission-related genes. The ability to modulate complex gene expression networks makes Semax a powerful research tool for investigating the molecular basis of neuroprotection and recovery mechanisms at the systems level.
The development of modified Semax derivatives, including N-Acetyl Semax, provides researchers with expanded tools for investigating peptide-mediated neuroprotection and cognitive enhancement. Laboratory studies suggest that these modified versions may offer enhanced pharmacokinetic properties while maintaining the core neuromodulatory and neuroprotective mechanisms of the parent compound. Research applications include investigations of cognitive function, neuroplasticity modulation, and neurotransmitter system regulation, particularly regarding dopaminergic pathway interactions. These derivative compounds offer researchers additional options for optimizing experimental conditions and investigating structure-activity relationships in peptide-based neuroprotection.
Safety Profile and Research Considerations
The safety profile of Semax in research applications demonstrates excellent tolerability with minimal adverse effects across multiple laboratory models and preclinical studies. Preclinical investigations document low toxicity profiles with no reports of serious adverse events following intranasal or subcutaneous administration in animal models. Laboratory studies indicate that Semax produces no debilitating effects on central nervous system function, no negative impact on cardiovascular parameters, and no evidence of drug dependence, addiction, or withdrawal syndrome in experimental settings. The compound's favorable safety profile extends to its lack of interference with the metabolism of other research compounds, making it suitable for combination studies and multi-target research protocols.
For research applications, investigators should consider several important factors when designing studies with Semax. The compound's rapid onset of action (effects measurable within 15 minutes) and regional specificity (preferential effects in basal forebrain versus cerebellum) make it suitable for both acute and chronic experimental protocols in laboratory models. Research dosing protocols typically utilize 50-250 μg/kg for animal studies, though investigators may require different dosing strategies depending on experimental objectives and model systems. The compound's stability and multiple administration routes (intranasal, intraperitoneal, subcutaneous) provide flexibility for different experimental designs while maintaining consistent bioavailability and central nervous system access.
Current regulatory considerations position Semax as a research chemical for laboratory and academic investigations, with regulatory agencies emphasizing the importance of maintaining research-only applications. The compound's status as an unregulated research chemical in the U.S. and EU allows investigators to utilize Semax for legitimate scientific investigations into neuroplasticity mechanisms, cognitive enhancement pathways, and neuroprotection research while adhering to institutional guidelines. Researchers should implement appropriate safety protocols and follow established procedures for handling research peptides, including proper storage conditions and disposal methods to maintain compound integrity and laboratory safety standards.
Current Research Directions and Future Applications
Contemporary research with Semax is expanding into novel applications that leverage the compound's unique combination of melanocortin receptor modulation, BDNF activation, and neuroprotective properties for investigating fundamental questions in neuroscience and molecular biology. Recent discoveries regarding Semax's targeting of the μ opioid receptor gene Oprm1 in spinal cord injury models have opened new research directions focused on understanding the interactions between melanocortin-derived peptides and opioid receptor systems. These molecular-level insights are driving investigations into how peptide-based interventions can achieve neuroprotective effects through previously unrecognized receptor targets and signaling pathways.
Advanced transcriptomic research utilizing RNA-Seq technology is providing researchers with unprecedented insights into Semax's genome-wide effects on gene expression patterns in laboratory models of neurological injury and dysfunction. Current investigations focus on understanding how Semax administration produces coordinated changes in inflammatory, neurotransmission, and neuroprotection gene networks, with particular emphasis on the temporal dynamics of these changes. These systems-level approaches are revealing new targets for research and providing insights into the complex molecular networks that underlie peptide-mediated neuroprotection and cognitive enhancement in experimental models.
Future research priorities include the development of enhanced Semax derivatives and combination protocols that leverage the compound's multi-target mechanisms for investigating specific aspects of neurobiological function. Research directions encompass the development of modified peptides with improved pharmacokinetic properties, investigation of synergistic effects with other research compounds, and exploration of novel delivery methods for targeted brain region studies. Advanced molecular techniques are being applied to understand Semax's long-term effects on neuroplasticity in laboratory models, including investigations of epigenetic modifications, synaptic remodeling, and cognitive reserve mechanisms. These mechanistic studies are expected to reveal new research applications and expand the utility of melanocortin-derived peptides in neuroscience research.
Conclusion: A Sophisticated Tool for Neurobiological Research
Semax represents a remarkable achievement in synthetic peptide design and neurobiological research tool development, offering investigators an unprecedented combination of melanocortin receptor modulation, BDNF activation, and neuroprotective properties within a single, well-characterized research compound. From its origins as an ACTH-derived analog to its current applications across multiple research disciplines, Semax has fundamentally advanced our understanding of peptide-mediated neuroplasticity while providing researchers with a sophisticated tool for investigating the complex relationships between melanocortin signaling, neurotrophic factors, and cognitive function in laboratory models. The compound's unique mechanism of action - combining receptor-specific modulation with transcriptional regulation and neuroprotective effects - positions it as an invaluable resource for advancing neuroscience research.
The compound's excellent safety profile in preclinical studies, rapid onset of action, and well-characterized molecular mechanisms provide researchers with confidence in experimental outcomes while ensuring reproducible and reliable research conditions. Whether applied to studies of neuroplasticity mechanisms, cognitive enhancement research, neuroprotection investigations, or neurotransmitter system interactions in laboratory settings, Semax offers the precision and reliability essential for advancing our understanding of brain function and developing novel research approaches. As research continues to reveal new molecular targets and mechanistic insights, this remarkable peptide remains at the forefront of neuroscience research, contributing to breakthrough discoveries that advance our understanding of neurobiological mechanisms and peptide-based research tools.
References
- Dolotov, O.V., et al. (2006). Semax, an analogue of adrenocorticotropin (4-10), binds specifically and increases levels of brain-derived neurotrophic factor protein in rat basal forebrain. Journal of Neurochemistry 96(6):1775-1783. https://doi.org/10.1111/j.1471-4159.2006.03658.x
- Eremin, K.O., et al. (2004). Semax, An ACTH(4-10) Analogue with Nootropic Properties, Activates Dopaminergic and Serotoninergic Brain Systems in Rodents. Neurochemical Research 31(2):137-148. https://doi.org/10.1007/s11064-005-8826-8
- Dmitrieva, V.G., et al. (2014). The peptide semax affects the expression of genes related to the immune and vascular systems in rat brain focal ischemia: genome-wide transcriptional analysis. BMC Genomics 15:228. https://doi.org/10.1186/1471-2164-15-228
- Manchenko, D.M., et al. (2020). Novel Insights into the Protective Properties of ACTH(4-7)PGP (Semax) Peptide at the Transcriptome Level Following Cerebral Ischaemia-Reperfusion in Rats. International Journal of Molecular Sciences 21(14):4834. https://doi.org/10.3390/ijms21144834
- Agapova, T.Y., et al. (2006). Semax, an analog of ACTH(4-10) with cognitive effects, regulates BDNF and trkB expression in the rat hippocampus. Brain Research 1114(1):126-133. https://doi.org/10.1016/j.brainres.2006.07.069
- Shadrina, M.I., et al. (2009). Comparison of the temporary dynamics of NGF and BDNF gene expression in rat hippocampus, frontal cortex, and retina under Semax action. Neurochemical Journal 3(3):193-200. https://doi.org/10.1134/S1819712409030071
- Kaplan, A.Y., et al. (2014). Semax, an ACTH 4-10 peptide analog with high affinity for brain binding sites and neuroprotective properties. Peptides 58:42-49. https://doi.org/10.1016/j.peptides.2014.05.018
- Stavchansky, V.V., et al. (2022). Development of Peptide Biopharmaceuticals in Russia. Pharmaceutics 14(4):716. https://doi.org/10.3390/pharmaceutics14040716
- Myasoedov, N.F., et al. (2001). Rapid induction of neurotrophin mRNAs in rat glial cell cultures by Semax, an adrenocorticotropic hormone analog. Neuroscience Letters 306(1-2):115-118. https://doi.org/10.1016/s0304-3940(01)01994-2
- Kondratenko, R.V., et al. (2019). Experimental Substantiation of Application of Semax as a Modulator of Immune Reaction on the Model of "Social" Stress. Bulletin of Experimental Biology and Medicine 167(1):72-76. https://doi.org/10.1007/s10517-019-04476-1
- University of Texas Rio Grande Valley. (2025). Research Colloquium: Semax for Parkinson's Neuroprotection: A Qualitative Review of Preclinical Evidence. ScholarWorks @ UTRGV. https://scholarworks.utrgv.edu/biosci_colloquium/2025
- PubMed Publication. (2025). Semax peptide targets the μ opioid receptor gene Oprm1 to promote deubiquitination and functional recovery after spinal cord injury in female mice. PubMed ID: 40692165. https://pubmed.ncbi.nlm.nih.gov/40692165/
- Russian Federation Government. (2011). Russian List of Vital & Essential Drugs. Approved December 7, 2011.
- FDA Database. (2024). Unregulated Research Chemical Status for Semax in United States. https://www.fda.gov/drugs/development-approval-process-drugs
- WADA. (2025). The Prohibited List 2025. World Anti-Doping Agency. https://www.wada-ama.org/sites/default/files/2024-09/2025list_en_final_clean_12_september_2024.pdf