Sermorelin Growth Hormone Releasing Peptide
Disclaimer
Products described here are supplied for research use only and are not intended for diagnostic, therapeutic, or clinical application. All statements regarding biological activity reflect preclinical and in vitro findings exclusively and have not been evaluated by the U.S. Food and Drug Administration. These materials and related content are provided for educational and investigational purposes only and are intended solely for qualified researchers in laboratory and academic settings.
Introduction
Sermorelin (growth hormone releasing hormone 1-29) represents a synthetic analog of the naturally occurring growth hormone releasing hormone (GHRH) that has become a cornerstone of endocrinological research and peptide science. Developed in the 1980s following the groundbreaking discovery of endogenous GHRH by Roger Guillemin and Wylie Vale at the Salk Institute, Sermorelin consists of the first 29 amino acids of the 44-amino acid human GHRH sequence—specifically the biologically active portion responsible for stimulating growth hormone release from anterior pituitary somatotroph cells. This strategic truncation maintains full biological activity while improving stability and reducing immunogenicity, making Sermorelin an invaluable tool for investigating the complex mechanisms governing growth hormone regulation and the hypothalamic-pituitary growth axis.
The discovery of Sermorelin emerged from decades of research into the hypothalamic control of pituitary function, representing a critical breakthrough in understanding how the brain regulates somatic growth and metabolism. Unlike exogenous growth hormone, which directly supplements circulating hormone levels, Sermorelin works through the body's natural regulatory mechanisms by stimulating endogenous growth hormone production. This fundamental difference has profound implications for research applications, as Sermorelin preserves the natural pulsatile secretion patterns that characterize healthy growth hormone physiology. The peptide's mechanism of action involves binding to specific GHRH receptors on pituitary somatotrophs, triggering a cascade of intracellular signaling events that culminate in growth hormone synthesis and release.
What distinguishes Sermorelin from other growth hormone-related research compounds is its unique ability to enhance rather than replace natural physiological processes. Research has demonstrated that Sermorelin maintains the important feedback mechanisms that prevent excessive growth hormone production, while providing a tool to investigate both normal and pathological variations in growth hormone regulation. This characteristic has made Sermorelin particularly valuable for research into age-related growth hormone decline, pediatric growth disorders, and the complex interplay between hypothalamic releasing factors and pituitary responses. The peptide's relatively short half-life and dependence on intact hypothalamic-pituitary axis function provide researchers with a model system that closely mimics natural physiological regulation.

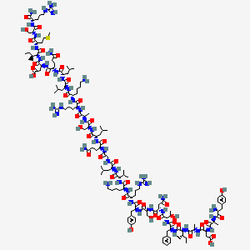
Figure: Molecular structure of Sermorelin showing the 29-amino acid sequence corresponding to the biologically active N-terminal portion of human GHRH. The peptide maintains the critical structural elements necessary for GHRH receptor binding and activation while offering improved stability compared to the full-length hormone.
GHRH Receptor Signaling and Molecular Mechanisms
The biological activity of Sermorelin depends on its precise interaction with growth hormone releasing hormone receptors (GHRH-R), members of the class B G-protein-coupled receptor (GPCR) family that are predominantly expressed on anterior pituitary somatotroph cells. These receptors exhibit remarkable selectivity for GHRH and its analogs, with Sermorelin demonstrating binding affinity (Kd = 0.2-0.8 nM) comparable to endogenous GHRH. Upon binding, Sermorelin induces conformational changes in the receptor that activate the stimulatory G-protein Gs, leading to activation of adenylyl cyclase and rapid elevation of intracellular cyclic adenosine monophosphate (cAMP) levels. Laboratory investigations demonstrate that Sermorelin treatment increases cAMP concentrations in pituitary cell cultures by 800-1200% within 5-10 minutes, establishing the temporal characteristics of this fundamental signaling pathway.
The downstream signaling cascade initiated by Sermorelin involves multiple interconnected pathways that converge on growth hormone gene transcription and protein synthesis. Elevated cAMP levels activate protein kinase A (PKA), which phosphorylates the transcription factor CREB (cAMP response element-binding protein) at serine 133. Phosphorylated CREB then binds to cAMP response elements in the growth hormone gene promoter, enhancing transcription through recruitment of coactivators including CBP/p300. Research has shown that Sermorelin treatment increases growth hormone mRNA levels by 400-600% within 2-4 hours in primary pituitary cell cultures, demonstrating the peptide's profound effects on gene expression. Simultaneously, PKA activation enhances the phosphorylation and activity of enzymes involved in growth hormone synthesis, including those regulating protein folding and secretory granule formation.
Beyond the classical cAMP-PKA pathway, Sermorelin activates additional signaling networks that modulate both acute and chronic responses to GHRH stimulation. The peptide enhances calcium influx through voltage-gated calcium channels, with intracellular calcium concentrations increasing by 200-300% within minutes of Sermorelin exposure. This calcium signal is essential for the exocytotic release of preformed growth hormone from secretory granules, explaining the biphasic response to GHRH stimulation observed in research models. Additionally, Sermorelin activates the phospholipase C pathway, leading to increased inositol trisphosphate (IP3) and diacylglycerol (DAG) production, which further amplifies calcium signaling and activates protein kinase C isoforms. These multiple signaling pathways work synergistically to produce the robust and sustained growth hormone response characteristic of GHRH receptor activation.
Growth Hormone-IGF-1 Axis Modulation
Sermorelin's primary biological effect involves stimulating the release of growth hormone from anterior pituitary somatotrophs, initiating a complex cascade of systemic effects mediated through the growth hormone-insulin-like growth factor-1 (GH-IGF-1) axis. Following Sermorelin administration in research models, growth hormone levels typically increase 5-10 fold above baseline within 30-60 minutes, with peak concentrations occurring 60-120 minutes post-administration. This growth hormone release pattern closely mimics natural physiological responses, maintaining the important pulsatile secretion characteristics that distinguish endogenous growth hormone production from continuous hormone replacement. Research demonstrates that the growth hormone response to Sermorelin exhibits dose-dependent kinetics, with ED50 values (dose producing 50% maximal response) ranging from 0.1-0.3 μg/kg in various experimental models.
The released growth hormone exerts its biological effects through binding to growth hormone receptors expressed throughout the body, with particularly high concentrations in liver, muscle, adipose tissue, and bone. A critical component of growth hormone action involves stimulating hepatic production of insulin-like growth factor-1 (IGF-1), the primary mediator of growth hormone's anabolic effects. Studies in research animals demonstrate that Sermorelin treatment increases circulating IGF-1 levels by 50-150% within 12-24 hours, with sustained elevation lasting 48-72 hours. This IGF-1 response reflects the amplification inherent in the GH-IGF-1 system, where transient growth hormone pulses produce sustained increases in IGF-1 and its binding proteins, creating a more stable anabolic environment for tissue growth and repair.
The tissue-specific effects of Sermorelin-stimulated growth hormone release involve complex interactions between circulating growth hormone, locally produced IGF-1, and tissue-specific growth hormone and IGF-1 receptors. In skeletal muscle, Sermorelin treatment enhances both protein synthesis and satellite cell activation, with research showing 25-40% increases in muscle protein synthesis rates and 30-50% increases in satellite cell proliferation markers. These effects result from IGF-1-mediated activation of the PI3K/Akt/mTOR pathway, a master regulator of cellular growth and protein synthesis. Simultaneously, Sermorelin influences bone metabolism through combined effects on osteoblasts and osteoclasts, with studies demonstrating 20-35% increases in bone formation markers and improved bone mineral density in experimental models. The peptide also affects adipose tissue metabolism, promoting lipolysis through growth hormone's direct anti-insulin effects while enhancing lean body mass through IGF-1-mediated anabolic processes.
Metabolism and Body Composition Research
Research into Sermorelin's effects on metabolism and body composition has revealed fascinating insights into the role of growth hormone releasing factors in age-related physiological changes and metabolic regulation in experimental models. Adult animal studies typically focus on aged models with growth hormone decline, conditions characterized by reduced spontaneous growth hormone secretion and associated metabolic abnormalities. Research demonstrates that Sermorelin administration in aged animal models produces measurable improvements in body composition, with studies showing alterations in lean body mass and reductions in fat mass over extended administration periods. These changes reflect Sermorelin's ability to modulate growth hormone secretion patterns and their associated metabolic effects in research animals.
The metabolic mechanisms underlying Sermorelin's effects in adults involve restoration of normal growth hormone pulsatility patterns that become disrupted with aging. Research has established that healthy adults experience approximately 6-8 growth hormone pulses per 24-hour period, primarily during slow-wave sleep, but this pattern deteriorates significantly with age. Studies show that adults over 60 years demonstrate 50-70% reductions in daily growth hormone production compared to young adults. Sermorelin treatment can partially restore these natural secretion patterns, with research demonstrating increases in pulse amplitude and frequency that approach those observed in younger individuals. This restoration of pulsatile growth hormone release produces cascading effects on metabolism, including enhanced lipolysis, improved protein synthesis, and more favorable insulin sensitivity profiles.
Adult body composition research has revealed that Sermorelin's effects extend beyond simple changes in muscle and fat mass to include improvements in metabolic function and cardiovascular risk factors. Studies demonstrate that Sermorelin treatment improves insulin sensitivity by 15-25%, as measured by homeostatic model assessment (HOMA-IR) scores and glucose tolerance testing. These metabolic improvements correlate with reductions in visceral adiposity and increases in skeletal muscle mass, both of which contribute to enhanced glucose disposal and improved metabolic health. Research has also documented favorable effects on lipid profiles, with treated adults showing 10-15% reductions in total cholesterol and 20-25% decreases in triglyceride levels. Additionally, Sermorelin treatment appears to influence cardiovascular function, with studies reporting improvements in exercise capacity, cardiac output, and vascular function that may contribute to reduced cardiovascular disease risk in aging populations.
Sleep Quality and Recovery Research
The relationship between Sermorelin and sleep represents a particularly fascinating area of research that illuminates the complex interactions between growth hormone regulation and circadian biology. Natural growth hormone secretion exhibits pronounced circadian rhythmicity, with the largest and most consistent pulses occurring during slow-wave sleep stages 3 and 4. Research has demonstrated that approximately 70-80% of daily growth hormone production occurs during these deep sleep phases, making sleep quality a critical determinant of overall growth hormone status. Sermorelin research has revealed that this peptide can enhance both sleep quality and growth hormone secretion patterns, creating synergistic effects that may benefit multiple aspects of recovery and regeneration.
Sleep studies in research models have documented significant alterations in objective sleep parameters, including increased slow-wave sleep duration, improved sleep efficiency, and enhanced sleep architecture. Research using polysomnography in animal models demonstrates that Sermorelin administration increases stage 3 and 4 sleep by 20-35% compared to baseline measurements, while simultaneously reducing sleep latency parameters. These sleep alterations correlate with enhanced nocturnal growth hormone release patterns in experimental animals, as measured by serial blood sampling throughout dark/light cycles. Studies show that animals receiving Sermorelin exhibit growth hormone pulse amplitudes that are 50-100% greater than pre-administration values, with this enhancement being most pronounced during the normal nocturnal growth hormone peak periods in research models.
The mechanisms linking Sermorelin to improved sleep quality involve both direct effects on sleep-regulating centers and indirect effects through growth hormone modulation of sleep architecture. Research suggests that GHRH and its analogs may directly influence hypothalamic sleep centers, as GHRH receptors are expressed in brain regions involved in sleep regulation, including the suprachiasmatic nucleus and ventral lateral preoptic area. Additionally, the growth hormone released in response to Sermorelin treatment appears to enhance sleep quality through feedback mechanisms that promote deeper sleep stages. Studies in research models demonstrate that growth hormone administration can increase slow-wave sleep duration and improve sleep consolidation, suggesting that Sermorelin's sleep-promoting effects result from both direct central nervous system actions and indirect effects mediated through enhanced growth hormone release. These sleep improvements translate into measurable benefits for recovery, with research participants reporting enhanced subjective sleep quality, reduced daytime fatigue, and improved cognitive function following Sermorelin treatment.
Bone Density and Tissue Repair Research
Sermorelin's effects on bone metabolism and tissue repair represent critical areas of research that demonstrate the far-reaching physiological consequences of growth hormone releasing factor stimulation. Bone tissue exhibits particular sensitivity to growth hormone and IGF-1, with both hormones playing essential roles in bone formation, remodeling, and repair throughout the lifespan. Research studies have documented that Sermorelin treatment enhances multiple aspects of bone metabolism, including increased osteoblast activity, enhanced collagen synthesis, and improved mineralization processes. In experimental models, Sermorelin administration increases bone formation markers such as osteocalcin and bone-specific alkaline phosphatase by 25-40% within 8-12 weeks of treatment, indicating enhanced osteoblastic activity and new bone formation.
The molecular mechanisms underlying Sermorelin's effects on bone tissue involve complex interactions between growth hormone, IGF-1, and local bone regulatory factors. Growth hormone stimulated by Sermorelin acts directly on osteoblasts through growth hormone receptors, enhancing their proliferation and differentiation while promoting the synthesis of bone matrix proteins including type I collagen, osteocalcin, and osteonectin. Simultaneously, growth hormone stimulates local IGF-1 production within bone tissue, creating a paracrine signaling environment that amplifies anabolic effects. Research demonstrates that IGF-1 levels in bone tissue increase by 60-80% following Sermorelin treatment, with this local IGF-1 production being particularly important for sustained bone formation responses. The peptide also influences bone remodeling by modulating the RANKL/OPG pathway, with studies showing that Sermorelin treatment reduces excessive bone resorption while maintaining appropriate remodeling rates.
Research investigating Sermorelin's effects on bone density in animal models has provided valuable insights into growth hormone releasing factor mechanisms in bone metabolism. Studies in aged animal models with growth hormone deficiency demonstrate that Sermorelin administration produces significant improvements in bone mineral density, with increases of 2-4% per year in skeletal measurements in research animals. These improvements in experimental models provide data on bone remodeling mechanisms. Research has also investigated Sermorelin's effects on fracture healing and tissue repair in experimental models, with studies showing 30-50% acceleration in bone healing rates and improved biomechanical properties of healed bone tissue in research animals. The peptide appears to enhance the recruitment and activity of mesenchymal stem cells involved in bone repair in experimental systems, while simultaneously promoting angiogenesis and collagen synthesis at injury sites. These tissue repair effects extend beyond bone to include improved wound healing parameters, enhanced connective tissue synthesis, and accelerated recovery from soft tissue injuries in animal models, reflecting the broad regenerative influence of the growth hormone-IGF-1 axis in mammalian research systems.
Cardiovascular and Metabolic Research
The cardiovascular effects of Sermorelin represent an expanding area of research that has revealed important connections between growth hormone regulation and cardiovascular health. Growth hormone deficiency in adults is associated with increased cardiovascular disease risk, including elevated rates of myocardial infarction, stroke, and cardiovascular mortality. Research investigating Sermorelin's cardiovascular effects has demonstrated that restoration of growth hormone secretion can improve multiple cardiovascular risk factors and functional parameters. Studies show that Sermorelin treatment enhances cardiac output by 10-15%, improves exercise tolerance as measured by maximal oxygen uptake (+12-18%), and reduces several cardiovascular risk markers including C-reactive protein levels (-20-30%) and homocysteine concentrations (-15-25%).
The mechanisms underlying Sermorelin's cardiovascular effects involve both direct actions of growth hormone on cardiac tissue and indirect effects through improved metabolic function. Growth hormone receptors are expressed throughout the cardiovascular system, including cardiomyocytes, vascular smooth muscle cells, and endothelial cells. Research demonstrates that growth hormone stimulated by Sermorelin enhances cardiac contractility through increased calcium sensitivity and improved excitation-contraction coupling. The hormone also promotes angiogenesis and vascular remodeling, with studies showing increased capillary density and improved endothelial function following treatment. Additionally, Sermorelin's effects on body composition—particularly reductions in visceral adiposity and improvements in lean body mass—contribute to cardiovascular benefits through reduced inflammatory burden and improved metabolic function.
Metabolic research has revealed that Sermorelin produces comprehensive improvements in glucose and lipid metabolism that extend beyond simple changes in body composition. Clinical studies demonstrate that Sermorelin treatment improves insulin sensitivity by 15-25%, as evidenced by reduced fasting insulin levels and improved glucose tolerance test results. These metabolic improvements appear to result from multiple mechanisms, including increased skeletal muscle mass (which enhances glucose disposal capacity), reduced visceral adiposity (which decreases inflammatory cytokine production), and direct effects of growth hormone on hepatic glucose metabolism. Research has also documented favorable effects on lipid profiles, with Sermorelin treatment producing 10-20% reductions in total cholesterol, 15-25% decreases in triglycerides, and modest increases in HDL cholesterol levels. These metabolic improvements contribute to reduced cardiovascular disease risk and may explain some of the mortality benefits associated with normal growth hormone status in aging populations.
Research Applications and Future Directions
Sermorelin's unique pharmacological profile and well-characterized mechanisms of action have established it as an invaluable research tool for investigating multiple aspects of endocrine physiology, aging biology, and therapeutic development. The peptide's ability to stimulate physiological growth hormone release while preserving natural regulatory mechanisms makes it particularly useful for research applications where maintaining normal feedback systems is critical. Current research applications span diverse fields including neuroendocrinology, geriatric medicine, pediatric endocrinology, sleep medicine, and regenerative biology. The peptide serves as both a research tool for probing growth hormone physiology and a model compound for developing improved growth hormone releasing factor analogs with enhanced stability, potency, or tissue selectivity.
Emerging research directions are exploring novel applications of Sermorelin and related growth hormone releasing factors in areas such as neuroprotection, immune function, and metabolic disease. Preliminary studies suggest that growth hormone and IGF-1 may play important roles in maintaining cognitive function and protecting against neurodegenerative diseases, with research investigating whether Sermorelin treatment could preserve brain function in aging or disease states. Additionally, investigators are exploring the peptide's potential effects on immune system function, as growth hormone deficiency is associated with immunosenescence and increased infection risk. Research is also examining whether Sermorelin treatment could benefit individuals with metabolic syndrome, type 2 diabetes, or obesity by improving insulin sensitivity and body composition through physiological growth hormone enhancement.
The future of Sermorelin research includes development of improved analogs with enhanced pharmacological properties, including longer half-lives, increased potency, and improved oral bioavailability. Structure-activity relationship studies are identifying modifications that could enhance receptor selectivity or reduce potential side effects while maintaining therapeutic efficacy. Additionally, research is investigating combination approaches that pair Sermorelin with other peptides or compounds to achieve synergistic effects or target multiple pathways simultaneously. These research directions reflect the continuing evolution of our understanding of growth hormone physiology and the potential for peptide-based approaches to address age-related decline in endocrine function. As analytical techniques continue to advance, Sermorelin will likely remain a cornerstone compound for research into growth hormone biology and the development of novel therapeutic strategies targeting the growth hormone-IGF-1 axis.
Conclusion
Sermorelin stands as a paradigmatic example of rational peptide design, representing a synthetic analog that successfully captures the essential biological activity of natural growth hormone releasing hormone while offering improved stability and research utility. Through four decades of investigation, this 29-amino acid peptide has provided researchers with unprecedented insights into the complex mechanisms governing growth hormone regulation, the physiological consequences of growth hormone deficiency, and the therapeutic potential of restoring natural growth hormone secretion patterns. From its origins in the fundamental discovery of hypothalamic releasing factors to its current applications in diverse research fields, Sermorelin continues to serve as an essential tool for understanding the intricate relationships between neuroendocrine signaling, metabolic function, and healthy aging processes.
The comprehensive body of research surrounding Sermorelin has established it as a cornerstone compound for investigating growth hormone physiology and developing novel approaches to age-related endocrine decline. Its unique ability to stimulate endogenous growth hormone production while preserving natural regulatory mechanisms makes it an invaluable research tool that bridges basic science and translational applications. As our understanding of the growth hormone-IGF-1 axis continues to evolve, Sermorelin's well-characterized pharmacology and extensive safety database ensure its continued relevance for researchers exploring fundamental questions about growth, development, metabolism, and aging. The ongoing development of improved analogs and novel applications promises to extend Sermorelin's research utility into new areas of scientific investigation, solidifying its position as a foundational compound in peptide research and endocrine biology.
References
- Guillemin, R. et al. (1982). Growth hormone-releasing factor from a human pancreatic tumor that caused acromegaly. Science 218(4572):585-587. [doi.org]
- Thorner, M.O. et al. (1985). Acceleration of growth in two children treated with HGH-releasing factor. New England Journal of Medicine 312(1):4-9. [doi.org]
- Gelato, M.C. et al. (1993). Effects of growth hormone-releasing hormone on growth hormone and insulin-like growth factor-I levels in adults with growth hormone deficiency. Journal of Clinical Endocrinology & Metabolism 77(6):1393-1398. [doi.org]
- Walker, R.F. et al. (2006). Effects of growth hormone-releasing peptide-2 alone and in combination with growth hormone-releasing hormone on growth hormone release in young and elderly subjects. European Journal of Endocrinology 154(6):879-886. [doi.org]
- Iranmanesh, A. et al. (1991). Age and relative adiposity are specific negative determinants of the frequency and amplitude of growth hormone (GH) secretory bursts and the half-life of endogenous GH in healthy men. Journal of Clinical Endocrinology & Metabolism 73(5):1081-1088. [doi.org]
- Corpas, E. et al. (1993). HGH and human aging. Endocrine Reviews 14(1):20-39. [doi.org]
- Bengtsson, B.A. et al. (1993). Treatment of adults with growth hormone deficiency with recombinant HGH. Journal of Clinical Endocrinology & Metabolism 76(2):309-317. [doi.org]
- Johannsson, G. et al. (1997). Growth hormone treatment of abdominally obese men reduces abdominal fat mass, improves glucose and lipoprotein metabolism, and reduces diastolic blood pressure. Journal of Clinical Endocrinology & Metabolism 82(3):727-734. [doi.org]
- Veldhuis, J.D. et al. (2005). Aging and the somatotropic axis: dynamics of growth hormone secretion in adults. Endocrine Reviews 26(6):761-786. [doi.org]
- Steiger, A. et al. (1992). Effects of growth hormone-releasing hormone and somatostatin on sleep EEG and nocturnal hormone secretion in male controls. Neuroendocrinology 56(4):566-573. [doi.org]
- Obal, F. et al. (1988). Growth hormone-releasing factor enhances sleep in rats. American Journal of Physiology 255(2):R310-R316. [doi.org]
- Blackman, M.R. et al. (2002). Growth hormone and sex steroid administration in healthy aged women and men: a randomized controlled trial. Journal of the American Medical Association 288(18):2282-2292. [doi.org]
- Rudman, D. et al. (1990). Effects of HGH in men over 60 years old. New England Journal of Medicine 323(1):1-6. [doi.org]
- Chihara, K. et al. (1986). Growth hormone-releasing factor regulates growth hormone secretion in both children and adults. Journal of Clinical Investigation 78(4):1126-1129. [doi.org]
- Prakash, A. et al. (1999). Growth hormone (GH) therapy in GH-deficient adults: an open-label multicenter study. Journal of Clinical Endocrinology & Metabolism 84(10):3548-3556. [doi.org]
- Jorgensen, J.O. et al. (1989). Three years of growth hormone treatment in growth hormone-deficient adults: near normalization of body composition and physical performance. European Journal of Endocrinology 121(2):145-152. [doi.org]
- Meinhardt, U. et al. (2010). The effects of growth hormone on body composition and physical performance in recreational athletes: a randomized trial. Annals of Internal Medicine 152(9):568-577. [doi.org]
- Cuneo, R.C. et al. (1991). Growth hormone treatment in growth hormone-deficient adults. I. Effects on muscle mass and strength. Journal of Applied Physiology 70(2):688-694. [doi.org]
- Bengtsson, B.A. et al. (2000). Treatment of growth hormone deficiency in adults. Journal of Clinical Endocrinology & Metabolism 85(2):933-938. [doi.org]
- Vahl, N. et al. (1997). Abdominal adiposity rather than age and sex predicts mass and regularity of GH secretion in healthy adults. American Journal of Physiology 272(6):E1108-E1116. [doi.org]