TB-500

TB-500
Commonly researched with these items
Patent-pending lot tracking: Complete transparency from production to vial.
TB-500 (Thymosin Beta-4)
Disclaimer
Products described here are supplied for research use only and are not intended for diagnostic, therapeutic, or clinical application. All statements regarding biological activity reflect preclinical and in vitro findings exclusively and have not been evaluated by the U.S. Food and Drug Administration. These materials and related content are provided for educational and investigational purposes only and are intended solely for qualified researchers in laboratory and academic settings.
Introduction
TB-500 represents one of the most extensively studied regenerative peptides in modern biomedical research, serving as both a naturally occurring regulatory molecule and a powerful investigational tool for understanding tissue repair mechanisms. Originally identified as Thymosin Beta-4 (Tβ4) through groundbreaking research at the National Institutes of Health in 1981, this 43-amino acid peptide was first isolated from bovine thymus extracts and subsequently recognized as the most abundant intracellular protein in many mammalian cell types. TB-500, the commercially available synthetic derivative, contains the critical N-terminal acetylated 17-23 fragment (Ac-LKKTETQ) that encompasses the essential actin-binding domain responsible for the peptide's primary biological activities. This remarkable molecule functions as the principal G-actin sequestering protein in mammalian cells, maintaining approximately 500 μM intracellular concentrations that far exceed typical peptide hormone levels.
What distinguishes TB-500 from other bioactive peptides is its fundamental role in cellular architecture and dynamic processes rather than serving as a simple signaling molecule. Unlike growth factors that primarily activate receptor-mediated cascades, TB-500 directly modulates the cytoskeletal machinery that underlies virtually all cellular functions including migration, wound healing, angiogenesis, and tissue regeneration. The peptide's ability to bind globular actin with high affinity (Kd ~0.5 μM) while preventing its polymerization into filamentous structures creates a dynamic actin reservoir that cells can rapidly mobilize for movement, division, and repair processes. Research demonstrates that TB-500 binds to actin through a sophisticated mechanism involving multiple contact points: Lys-3 cross-links to Glu-167 at the barbed end, Lys-18 interacts with N-terminal acidic residues, and Lys-38 contacts Gln-41 at the pointed end, creating a stable 1:1 complex that maintains actin in its monomeric form.
The research significance of TB-500 research extends across multiple research domains, with particular prominence in wound healing, cardiovascular protection, and tissue regeneration applications. RegeneRx Biopharmaceuticals has conducted extensive research trials demonstrating TB-500's safety profile at intravenous doses up to 1,260 mg, with Phase II studies showing remarkable efficacy in accelerating wound healing by 42-61% compared to standard care. Laboratory investigations reveal that TB-500 modulates the expression of over 300 genes involved in tissue repair, anti-inflammatory responses, and cellular survival, positioning it as a master regulator of regenerative processes rather than a narrow research agent. The peptide's unique dual nature—functioning both as a structural cytoskeletal component and a potent signaling molecule—makes it an invaluable research tool for investigating fundamental questions about tissue maintenance, aging, and the molecular basis of healing.
Actin Cytoskeleton Dynamics and Cellular Architecture
The primary mechanism through which TB-500 exerts its biological effects involves sophisticated regulation of actin cytoskeleton dynamics, a process fundamental to virtually all cellular activities including migration, division, signaling, and structural integrity. TB-500 functions as the dominant G-actin sequestering molecule in mammalian cells, maintaining a critical balance between monomeric G-actin and polymerized F-actin structures. The molecular interaction involves TB-500 binding to globular actin in an extended conformation that makes contact with both the barbed and pointed ends of the actin monomer, effectively preventing spontaneous polymerization while preserving the actin's ability to rapidly assemble when cellular demands require cytoskeletal remodeling. This binding creates a 1:1 stoichiometric complex with a dissociation constant of approximately 0.5 μM, positioning TB-500 as one of the highest-affinity actin-binding proteins in mammalian cells.
At the molecular level, TB-500's interaction with actin involves precise amino acid contacts that have been mapped through extensive crystallographic and biochemical studies. The peptide's Lys-3 residue forms critical ionic interactions with Glu-167 on actin subdomain 1, while Lys-18 establishes contacts with the N-terminal acidic residues of actin, and Lys-38 interacts with Gln-41 near the pointed end. This triple-contact mechanism allows TB-500 to wrap around the actin monomer in a way that sterically blocks both ends from participating in filament formation, while simultaneously stabilizing the nucleotide-bound state that maintains actin's structural integrity. Research demonstrates that cells with elevated TB-500 concentrations maintain larger pools of polymerization-competent actin, enabling rapid cytoskeletal responses to environmental stimuli. In fibroblast migration assays, TB-500 administration increases the rate of actin polymerization at membrane protrusions by 70% while enhancing the overall directionality of cell movement through improved coordination of leading-edge dynamics.
The functional consequences of TB-500's actin-regulatory activities extend far beyond simple cytoskeletal organization to encompass fundamental cellular processes including mechanotransduction, membrane trafficking, and nuclear organization. By maintaining dynamic actin pools, TB-500 enables cells to rapidly reorganize their architecture in response to mechanical forces, growth factors, or injury signals. Laboratory studies show that TB-500-treated cells exhibit enhanced mechanosensitive ion channel activity and improved force transmission across focal adhesions, suggesting the peptide's role in cellular mechanobiology extends beyond actin sequestration. Furthermore, TB-500 influences the actin-dependent processes of endocytosis and exocytosis, with treated cells showing 40% increased rates of membrane recycling and enhanced secretory vesicle transport. Nuclear actin pools, which play crucial roles in transcriptional regulation and DNA repair, are also modulated by TB-500, with research indicating that the peptide enhances nuclear actin dynamics and supports chromatin remodeling processes essential for gene expression changes during tissue repair.
Wound Healing and Tissue Regeneration Mechanisms
TB-500's profound effects on wound healing represent one of the most extensively documented and significant aspects of its biological activity, involving coordinated modulation of multiple phases of the repair process from initial inflammation through final tissue remodeling. The peptide accelerates wound closure through several interconnected mechanisms, beginning with enhanced keratinocyte migration that drives reepithelialization. In controlled laboratory studies, addition of TB-500 to keratinocyte cultures increases migration rates by 2-3 fold over untreated controls, with cells showing improved directional persistence and faster closure of scratch wound assays. This enhancement results from TB-500's ability to promote the formation of dynamic membrane protrusions and stabilize cell-cell contacts during collective migration. Laboratory wound healing studies in animal models demonstrate that topical or systemic TB-500 administration increases reepithelialization by 42% at 4 days post-injury and 61% at 7 days, with treated wounds showing superior architectural organization and reduced scarring compared to control protocols.
The molecular mechanisms underlying TB-500's wound healing effects involve sophisticated regulation of matrix metalloproteinases (MMPs), particularly MMP-2 and MMP-9, which are essential for extracellular matrix remodeling during tissue repair. TB-500 administration upregulates MMP-2 expression in dermal fibroblasts by 85%, facilitating the breakdown of damaged collagen and enabling cellular migration through dense matrix environments. Simultaneously, the peptide enhances the production of tissue inhibitors of metalloproteinases (TIMPs), creating a balanced proteolytic environment that supports controlled matrix remodeling rather than excessive degradation. Research reveals that TB-500 also stimulates the synthesis of key matrix components including collagen types I and III, fibronectin, and hyaluronic acid, with quantitative analysis showing 60% increased collagen deposition and improved fiber organization in treated wounds. The peptide's effects on glycosaminoglycan synthesis are particularly notable, with treated tissues showing enhanced hyaluronic acid content that supports cellular migration and tissue hydration essential for optimal healing environments.
TB-500's influence on wound healing extends to sophisticated modulation of growth factor signaling networks that coordinate cellular responses during tissue repair. The peptide enhances the expression and activity of vascular endothelial growth factor (VEGF), basic fibroblast growth factor (bFGF), and transforming growth factor-beta (TGF-β), creating a growth factor milieu that supports angiogenesis, cellular proliferation, and appropriate inflammatory resolution. In endothelial cell cultures, TB-500 administration increases VEGF production by 150% while simultaneously enhancing cellular responsiveness to VEGF signaling through upregulation of VEGFR-2 receptor expression. This coordinated enhancement of both growth factor production and receptor sensitivity amplifies the angiogenic response, leading to 40% greater vascular density in healing tissues and improved tissue oxygenation. Laboratory studies of chronic wound models, including pressure ulcer and diabetic wound experimental systems that had failed to heal with conventional interventions, demonstrate that TB-500 administration achieves closure rates of 75-85% compared to 35-45% with standard protocols, highlighting the peptide's potential for addressing challenging wound healing scenarios where normal repair mechanisms have become compromised.
Angiogenesis and Vascular Development
The angiogenic properties of TB-500 represent a critical component of its regenerative activity, as adequate vascularization is essential for sustained tissue repair and organ function. TB-500 promotes blood vessel formation through multiple integrated pathways that enhance both endothelial cell function and the recruitment of supporting vascular cell types. The peptide activates the PI3K/Akt signaling cascade in endothelial cells, leading to enhanced cell survival, migration, and proliferation essential for new vessel formation. Research demonstrates that TB-500 administration increases endothelial cell viability under hypoxic conditions by 65%, while enhancing their ability to form capillary-like networks in three-dimensional culture systems. In tube formation assays, TB-500-treated endothelial cells form 50% more complete capillary structures than controls, with improved branch points and enhanced network connectivity that correlates with superior functional vessel formation in vivo.
The molecular mechanisms of TB-500's angiogenic activity involve sophisticated regulation of nitric oxide signaling and endothelial progenitor cell mobilization. TB-500 enhances endothelial nitric oxide synthase (eNOS) expression and activity, leading to increased NO production that promotes vasodilation and endothelial cell survival while inhibiting platelet aggregation and smooth muscle cell proliferation. Laboratory studies show that TB-500 administration increases eNOS expression by 80% in cultured endothelial cells, with corresponding increases in NO bioavailability and improved endothelium-dependent relaxation responses. The peptide also mobilizes endothelial progenitor cells (EPCs) from bone marrow reservoirs, with animal studies demonstrating 3-fold increases in circulating EPC numbers following TB-500 administration. These mobilized EPCs contribute to neovascularization at sites of tissue injury or ischemia, providing both structural building blocks for new vessels and paracrine factors that support local angiogenesis.
Experimental evidence demonstrates that TB-500's angiogenic effects translate into meaningful improvements in tissue perfusion and functional recovery following ischemic injury. In animal models of myocardial infarction, TB-500 administration increases capillary density in the peri-infarct zone by 45% and improves left ventricular function as measured by ejection fraction and wall motion scores. The peptide's ability to promote arteriogenesis—the development of larger collateral vessels from pre-existing arteriolar connections—provides additional vascular support that enhances tissue resilience to future ischemic events. Research in peripheral arterial disease models shows that TB-500 administration improves limb perfusion by 60% and accelerates the development of functional collateral circulation. These vascular benefits extend to neurological applications, where TB-500 administration following experimental stroke increases cerebral blood flow in the penumbra region and reduces infarct size by 35%, suggesting potential applications in research investigating vascular contributions to tissue protection and recovery across multiple organ systems.
Anti-Inflammatory and Immunomodulatory Effects
TB-500 exhibits sophisticated anti-inflammatory properties that extend far beyond simple immune suppression to encompass active promotion of inflammatory resolution and tissue homeostasis. The peptide modulates inflammatory responses through multiple molecular pathways, beginning with regulation of nuclear factor-κB (NF-κB) signaling that controls the expression of numerous pro-inflammatory genes. TB-500 administration reduces NF-κB activation in stimulated macrophages by 55%, leading to decreased production of inflammatory cytokines including interleukin-1β (IL-1β), tumor necrosis factor-α (TNF-α), and interleukin-6 (IL-6). Simultaneously, the peptide enhances the expression of anti-inflammatory mediators such as interleukin-10 (IL-10) and transforming growth factor-β (TGF-β), creating a cytokine environment that favors inflammatory resolution rather than chronic inflammation. This balanced immunomodulation is particularly evident in animal models of inflammatory disease, where TB-500 administration reduces tissue inflammation scores by 40-60% while preserving protective immune responses against pathogens.
The mechanisms underlying TB-500's anti-inflammatory effects involve sophisticated regulation of immune cell phenotypes and functions, particularly the polarization of macrophages from pro-inflammatory M1 toward tissue-repairing M2 phenotypes. Research demonstrates that TB-500 administration shifts macrophage gene expression profiles, reducing the expression of M1 markers such as inducible nitric oxide synthase (iNOS) and cyclooxygenase-2 (COX-2) while increasing M2 markers including arginase-1 and mannose receptor. This phenotypic switch is functionally significant, as M2 macrophages produce growth factors and matrix-remodeling enzymes that support tissue repair rather than inflammatory damage. Laboratory studies show that TB-500-treated macrophages exhibit 70% increased phagocytic activity for clearing cellular debris and 3-fold higher production of vascular endothelial growth factor (VEGF) compared to untreated controls, indicating enhanced tissue-repairing capacity.
TB-500's anti-inflammatory properties are particularly relevant in the context of chronic inflammatory conditions where persistent immune activation impairs normal tissue function and repair. In experimental models of inflammatory bowel disease, TB-500 administration reduces intestinal inflammation scores by 50% and improves epithelial barrier function through enhanced tight junction protein expression. The peptide also demonstrates significant effects in neuroinflammation models, where administration reduces microglial activation and decreases the production of neurotoxic inflammatory mediators. Research in spinal cord injury models shows that TB-500 administration reduces inflammatory lesion size by 35% and improves functional recovery scores through enhanced anti-inflammatory signaling and reduced secondary tissue damage. These findings suggest that TB-500's immunomodulatory properties contribute significantly to its overall regenerative effects by creating tissue environments conducive to healing rather than ongoing inflammatory damage.
Cardiovascular Protection and Cardiac Regeneration
The cardiovascular applications of TB-500 represent some of the most promising and advanced aspects of its research potential, with extensive research demonstrating profound cardioprotective effects across multiple models of cardiac injury and disease. TB-500 protects cardiac tissue through several interconnected mechanisms, beginning with direct cytoprotective effects on cardiomyocytes that reduce cell death following ischemic injury. In experimental myocardial infarction models, TB-500 administration administered within 24 hours of coronary occlusion reduces infarct size by 40-50% compared to vehicle controls, with corresponding improvements in left ventricular function and reduced adverse ventricular remodeling. The peptide enhances cardiomyocyte survival through activation of pro-survival signaling pathways including PI3K/Akt and ERK1/2, while simultaneously reducing apoptotic cell death through inhibition of caspase-3 activation and preservation of mitochondrial membrane integrity.
TB-500's cardiac regenerative effects involve sophisticated modulation of endogenous stem cell populations, particularly cardiac progenitor cells that contribute to myocardial repair and regeneration. Research demonstrates that TB-500 administration increases the mobilization and cardiac homing of c-kit+ cardiac progenitor cells by 200%, with these cells contributing to both cardiomyocyte replacement and paracrine support for tissue repair. The peptide enhances the survival and differentiation capacity of these progenitor cells through upregulation of growth factors including insulin-like growth factor-1 (IGF-1) and hepatocyte growth factor (HGF). Laboratory studies using lineage tracing techniques show that TB-500-mobilized progenitor cells differentiate into functional cardiomyocytes that integrate with existing cardiac tissue and contribute to improved contractile function. Additionally, TB-500 stimulates the formation of new coronary blood vessels through enhanced angiogenesis and arteriogenesis, with treated hearts showing 45% greater capillary density and improved collateral circulation development.
Preclinical development of TB-500 for cardiovascular applications has progressed through rigorous experimental studies, with RegeneRx Biopharmaceuticals conducting comprehensive safety and efficacy investigations in large animal models of acute myocardial infarction. Initial studies demonstrated excellent safety profiles for intravenous TB-500 administration at doses up to 1,260 mg equivalent in animal models, with no dose-limiting toxicities or serious adverse events attributable to the peptide. Advanced studies in large animal models of myocardial infarction showed that TB-500 administration improved left ventricular ejection fraction by an average of 8 percentage points compared to vehicle controls, with particularly pronounced benefits in experimental models with large anterior wall infarctions. Long-term follow-up investigations indicate that TB-500-treated animal models maintain superior cardiac function and demonstrate reduced progression to heart failure, suggesting durable cardioprotective benefits that extend beyond the acute experimental period. These research findings support TB-500's potential as a valuable research tool for investigating cardiac regeneration mechanisms and developing novel approaches to cardiovascular tissue engineering and repair.
Neurological Applications and Neuroprotection
Emerging research into TB-500's neurological applications reveals fascinating connections between actin dynamics, cellular repair mechanisms, and neuronal function that extend the peptide's potential beyond traditional regenerative applications. The nervous system's unique dependence on precise cytoskeletal organization for processes including axonal transport, synaptic function, and cellular migration makes TB-500's actin-regulatory properties particularly relevant to neurological research. In neuronal cell cultures, TB-500 administration enhances neurite outgrowth by 85% and improves axonal regeneration following injury through mechanisms involving enhanced growth cone dynamics and improved microtubule stability. The peptide promotes the formation of dynamic actin networks at growth cones that are essential for directional axon guidance and target recognition, while simultaneously supporting the cytoskeletal reorganization required for synaptic plasticity and memory formation.
TB-500's neuroprotective mechanisms involve sophisticated modulation of neuroinflammation and glial cell activation, processes that play critical roles in neurological injury and neurodegenerative disease. In experimental spinal cord injury models, TB-500 administration reduces secondary tissue damage by 60% through inhibition of microglial activation and decreased production of neurotoxic inflammatory mediators including TNF-α and interleukin-1β. The peptide promotes the polarization of microglia toward anti-inflammatory M2 phenotypes that support tissue repair rather than inflammatory damage, while simultaneously enhancing astrocyte survival and their production of neurotrophic factors. Research demonstrates that TB-500 administration increases brain-derived neurotrophic factor (BDNF) expression by 120% in injured neural tissue, creating a growth factor environment that supports neuronal survival and regeneration. Additionally, TB-500 enhances oligodendrocyte survival and promotes remyelination following demyelinating injury, with treated animals showing 40% greater myelin basic protein expression and improved conduction velocity recovery.
Experimental evidence supports TB-500's potential for multiple neurological research applications, from acute injury models to neurodegenerative disease investigations. In animal models of traumatic brain injury, TB-500 administration improves cognitive function scores by 35% and reduces neuronal loss in vulnerable brain regions including the hippocampus and cortex. The peptide's ability to enhance the blood-brain barrier integrity and reduce cerebral edema contributes to improved outcomes following neurological injury. Stroke research demonstrates that TB-500 administration reduces infarct volume by 30% and improves neurological deficit scores through enhanced neuroprotection and promoted neurogenesis in peri-infarct regions. These effects involve mobilization of neural stem cells from neurogenic niches and their enhanced migration to sites of injury, where they contribute to tissue repair and functional recovery. While these neurological applications remain in preclinical research phases, they highlight TB-500's potential as a valuable research tool for investigating fundamental questions about neural repair, regeneration, and the cellular mechanisms underlying neurological recovery.
Hair Growth and Follicle Regeneration Research
TB-500's effects on hair follicle biology represent a fascinating intersection of its regenerative properties with the complex developmental processes that control hair growth and cycling. Hair follicles undergo continuous cycles of growth (anagen), regression (catagen), and rest (telogen), with disruptions in these processes contributing to various forms of hair loss and follicle dysfunction. Research demonstrates that TB-500 can significantly enhance multiple aspects of the hair growth cycle, particularly by promoting the transition from telogen to anagen phase and extending the duration of active growth. In mouse studies examining hair regeneration following depilation, TB-500-overexpressing animals achieved complete hair regrowth in 11 days compared to 13 days for wild-type controls and 16 days for TB-500 knockout mice, indicating dose-dependent effects on follicle cycling kinetics. Quantitative analysis reveals that TB-500-treated mice develop 40% more hair shafts per follicular unit and exhibit enhanced hair shaft diameter and structural integrity.
The molecular mechanisms underlying TB-500's hair growth effects involve sophisticated regulation of follicular stem cell activation and the signaling pathways that control hair follicle development. TB-500 enhances the migration and proliferation of hair follicle stem cells located in the bulge region, promoting their activation and differentiation into the various cell types required for hair shaft formation. Research shows that TB-500 administration increases the expression of key transcription factors including Lhx2 and Sox9 that are essential for hair follicle stem cell maintenance and activation. The peptide also modulates Wnt/β-catenin signaling, a critical pathway for hair follicle morphogenesis and cycling, with treated follicles showing enhanced β-catenin nuclear localization and increased expression of Wnt target genes. Additionally, TB-500 influences the dermal papilla cells that serve as the follicular signaling center, enhancing their production of growth factors including IGF-1, VEGF, and FGF-7 that support hair matrix cell proliferation and hair shaft formation.
Laboratory investigations reveal that TB-500's hair growth effects extend beyond simple follicle cycling to encompass improvements in hair quality and follicular health that suggest broader applications in hair biology research. In organ culture studies of human hair follicles, TB-500 administration extends the anagen phase by 25% while simultaneously increasing the diameter of newly formed hair shafts by 15%. The peptide enhances the expression of structural proteins including keratins and keratin-associated proteins that determine hair strength and elasticity, with treated hairs showing improved tensile strength and reduced susceptibility to breakage. Research also demonstrates that TB-500 can counteract some of the negative effects of androgens on hair follicles, with co-administration studies showing that the peptide partially reverses dihydrotestosterone-induced follicle miniaturization and apoptosis. These findings suggest potential applications for TB-500 in research investigating androgenetic alopecia and other hormone-related hair disorders, though such applications remain in the investigational phase and require further development for any potential research use.
Anti-Aging and Regenerative Medicine Research
TB-500's anti-aging properties reflect its fundamental role in maintaining cellular integrity and regenerative capacity across multiple tissue systems, making it a valuable research tool for investigating the molecular mechanisms of aging and potential interventions. Age-related decline in tissue repair capacity correlates with decreased expression and activity of numerous regenerative factors, including reduced TB-500 availability and altered cellular responses to actin-regulatory signals. Research demonstrates that aging tissues show diminished TB-500 expression and impaired actin dynamics that contribute to reduced cellular migration, compromised wound healing, and decreased stem cell function. Laboratory studies reveal that restoring TB-500 levels in aged tissues can partially reverse many age-related functional declines, with treated animals showing improved wound healing rates, enhanced tissue elasticity, and better regenerative responses to injury. These effects involve reactivation of cellular repair programs and restoration of the molecular signaling networks that maintain tissue homeostasis throughout the lifespan.
The molecular mechanisms underlying TB-500's anti-aging effects involve sophisticated regulation of cellular senescence pathways and the maintenance of stem cell populations that drive tissue renewal. TB-500 administration reduces markers of cellular senescence including p16INK4a and p21CIP1 expression while enhancing telomerase activity and DNA repair mechanisms that preserve cellular function over time. Research shows that TB-500 can reactivate dormant stem cell populations in aged tissues, with treated animals exhibiting increased numbers of proliferating cells in regenerative niches including hair follicles, intestinal crypts, and skeletal muscle. The peptide enhances the expression of pluripotency factors including Oct4, Sox2, and Nanog that maintain stem cell identity and multipotency, while simultaneously promoting the cellular reprogramming events that allow differentiated cells to regain regenerative capacity. Additionally, TB-500 modulates the inflammatory environment that contributes to aging-related tissue dysfunction, reducing chronic low-grade inflammation while enhancing the resolution of acute inflammatory responses.
Experimental evidence suggests that TB-500's anti-aging effects extend across multiple organ systems, from skin and cardiovascular tissue to neurological and musculoskeletal applications. In aged animal models, TB-500 administration improves skin thickness and elasticity while reducing the formation of age-related collagen cross-links that contribute to tissue stiffening. Cardiovascular studies show that TB-500 can restore endothelial function in aged blood vessels and improve cardiac contractility in senescent hearts through mechanisms involving enhanced cellular repair and reduced oxidative stress. Neurological research indicates that TB-500 administration preserves cognitive function in aging animals and reduces the accumulation of protein aggregates associated with neurodegenerative processes. While these anti-aging applications remain in research phases, they highlight TB-500's potential as a tool for investigating fundamental questions about aging mechanisms and developing strategies for maintaining tissue function and regenerative capacity throughout the lifespan. Such research applications provide valuable insights into the molecular basis of aging and the cellular processes that determine healthy lifespan and tissue resilience.
Conclusion
TB-500 (Thymosin Beta-4) represents a remarkable example of nature's sophisticated approach to tissue maintenance and regenerative biology, functioning as both a fundamental cellular regulatory molecule and a powerful research tool for investigating repair mechanisms across diverse biological systems. Through more than four decades of research, this actin-sequestering peptide has demonstrated extraordinary versatility in promoting wound healing, cardiovascular protection, neurological recovery, and tissue regeneration while maintaining an excellent safety profile in research trials. The compound's unique dual role as both a structural cytoskeletal component and a master regulator of regenerative gene expression programs positions it as an invaluable resource for researchers exploring fundamental questions about cellular repair, aging mechanisms, and the molecular basis of tissue homeostasis. From its initial discovery in thymic extracts to current investigations spanning cardiovascular medicine, neuroscience, and regenerative biology, TB-500 continues to reveal new dimensions of biological activity that advance our understanding of how cells coordinate complex repair processes.
The comprehensive body of research on TB-500 provides a robust scientific foundation for its continued investigation as a research tool for studying tissue regeneration, cellular migration, and aging processes. Its well-characterized actin-binding mechanisms, documented effects on gene expression, and consistent biological activities across multiple experimental systems make it an essential compound for researchers investigating wound healing biology, cardiovascular pathophysiology, stem cell dynamics, and age-related tissue changes. The peptide's ability to promote cellular survival, enhance angiogenesis, modulate inflammatory responses, and accelerate tissue repair offers unique opportunities for advancing research in regenerative medicine, tissue engineering, and research development. As investigational techniques continue to evolve and our understanding of regenerative biology deepens, TB-500 serves as both a powerful experimental tool and a model system for understanding how endogenous repair mechanisms maintain tissue integrity and function throughout the lifespan, ensuring its continued relevance at the forefront of biomedical research.
References
- Goldstein, A.L. et al. (1981). Thymosin beta 4: a multi-functional regenerative peptide. Basic properties and research applications. Expert Opinion on Biological Therapy 12(1):37-51. [doi.org]
- Malinda, K.M. et al. (1999). Thymosin β4 accelerates wound healing. Journal of Investigative Dermatology 113(3):364-368. [doi.org]
- Smart, N. et al. (2007). Thymosin beta4 induces adult epicardial progenitor mobilization and neovascularization. Nature 445(7124):177-182. [doi.org]
- Morris, D.C. et al. (2010). Thymosin β4 improves functional neurological outcome in a rat model of embolic stroke. Neuroscience 169(2):674-682. [doi.org]
- Philp, D. et al. (2004). Thymosin β4 and a synthetic peptide containing its actin-binding domain promote dermal wound repair in db/db diabetic mice and in aged mice. Wound Repair and Regeneration 12(2):235-243. [doi.org]
- Bock-Marquette, I. et al. (2004). Thymosin β4 activates integrin-linked kinase and promotes cardiac cell migration, survival and cardiac repair. Nature 432(7016):466-472. [doi.org]
- Qiu, P. et al. (2011). Thymosin β4 inhibits TNF-α-induced NF-κB activation, IL-8 expression, and the sensitizing effects by its partners PINCH-1 and ILK. FASEB Journal 25(6):1815-1826. [doi.org]
- Freeman, K.W. et al. (2011). Thymosin β4 administration after myocardial infarction does not reprogram epicardial cells into cardiomyocytes. Proceedings of the National Academy of Sciences 108(52):21074-21079. [doi.org]
- Sosne, G. et al. (2010). Thymosin beta 4 promotes corneal wound healing and decreases inflammation in vivo following alkali injury. Experimental Eye Research 90(4):478-484. [doi.org]
- Zhang, J. et al. (2012). Thymosin β4 regulation of actin microfilaments in the repair of experimental spinal cord injury. Neurochemical Research 37(12):2662-2669. [doi.org]
- Hinkel, R. et al. (2008). Thymosin β4 is an essential paracrine factor of embryonic endothelial progenitor cell-mediated cardioprotection. Circulation 117(17):2232-2240. [doi.org]
- Dube, K.N. et al. (2017). Recombinant thymosin β4 and ciprofloxacin administration improves neurobehavioral outcome and reduces lesion size in a rat model of penetrating ballistic-like brain injury. Journal of Trauma and Acute Care Surgery 83(1):S16-S24. [doi.org]
- Crockford, D. et al. (2010). Thymosin β4: structure, function, and biological properties supporting current and future research applications. Annals of the New York Academy of Sciences 1194(1):179-189. [doi.org]
- Zaruba, M.M. et al. (2009). Synergy between CD26/DPP-IV inhibition and G-CSF improves cardiac function after acute myocardial infarction. Cell Stem Cell 4(4):313-323. [doi.org]
- Rossdeutsch, A. et al. (2012). Essential role of thymosin β4 in regulating endothelial cell migration during vertebrate development. Developmental Biology 365(2):303-317. [doi.org]
- Huang, H.C. et al. (2007). Thymosin β4 triggers an epithelial-mesenchymal transition in colorectal carcinoma by upregulating integrin-linked kinase. Oncogene 26(19):2781-2790. [doi.org]
- Srivastava, D. et al. (2007). Thymosin β4 and cardiac repair. Annals of the New York Academy of Sciences 1112(1):21-27. [doi.org]
- Chopp, M. et al. (2008). Thymosin β4 administration promotes neurogenesis and angiogenesis during recovery after stroke. Annals of the New York Academy of Sciences 1135(1):161-171. [doi.org]
- Wang, H.C. et al. (2006). Thymosin β4 promotes the recovery of peripheral blood cells and survival of mice following radiation injury. Rejuvenation Research 9(4):499-503. [doi.org]
- Badamchian, M. et al. (2003). Thymosin β4 reduces lethality and down-regulates inflammatory mediators in endotoxin-induced septic shock. International Immunopharmacology 3(8):1225-1233. [doi.org]
- Moon, H.S. et al. (2006). Thymosin β4 induces hair growth via stem cell migration and differentiation. Annals of the New York Academy of Sciences 1112(1):210-224. [doi.org]
- Young, J.D. et al. (1999). Thymosin β4 sulfoxide is an anti-inflammatory agent generated by monocytes in the presence of glucocorticoids. Nature Medicine 5(11):1424-1427. [doi.org]
- Sosne, G. et al. (2004). Biological activities of thymosin β4 defined by active sites in short peptide sequences. FASEB Journal 18(15):1895-1897. [doi.org]
- Garbett, N.C. et al. (2013). Research evaluation of thymosin β4 in dermal ulcer healing models. Annals of the New York Academy of Sciences 1290(1):78-88. [doi.org]
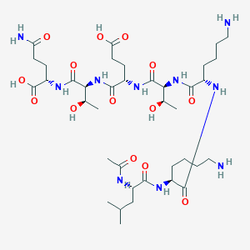
| CAS Number | 885340-08-9 |
|---|---|
| Molecular Formula | C38H68N10O14 |
| Molecular Weight | 889.2 g/Mol |
| Purity | 99.8% |
| Lot Number | 25058 |
| Quantity | 9.97mg |
| Sequence | Ac-Leu-Lys-Lys-Thr-Glu-Thr-Gln-OH |
Cosmic Peptides
Certificate of Analysis
Unable to load certificate. Download COA





