Thymalin

Thymalin
Commonly researched with these items
Patent-pending lot tracking: Complete transparency from production to vial.
Thymalin Thymic Peptide Complex
Disclaimer
Products described here are supplied for research use only and are not intended for diagnostic, therapeutic, or clinical application. All statements regarding biological activity reflect preclinical and in vitro findings exclusively and have not been evaluated by the U.S. Food and Drug Administration. These materials and related content are provided for educational and investigational purposes only and are intended solely for qualified researchers in laboratory and academic settings.
Introduction
Thymalin represents a pioneering achievement in immunogerontology research, emerging from the groundbreaking work of Professor Vladimir Khavinson and his colleagues at the Military Medical Academy in St. Petersburg during the 1980s and 1990s. This complex polypeptide preparation, extracted from the thymus glands of young calves, was developed as part of the revolutionary Russian bioregulatory peptide program that sought to identify and harness the body's natural regulatory mechanisms for research applications. Unlike single-peptide compounds, Thymalin represents a sophisticated mixture of thymic peptides with molecular weights below 10 kilodaltons, containing multiple bioactive sequences that work synergistically to modulate immune system function and combat the age-related decline in immune competence known as immunosenescence.
The development of Thymalin emerged from fundamental discoveries about the critical role of the thymus gland in immune system regulation and the devastating consequences of its age-related involution. Research had established that the thymus, a small organ located behind the sternum, serves as the primary site for T-cell development and immune system education during youth, but undergoes progressive shrinkage and functional decline beginning in adolescence. This thymic involution leads to reduced production of naive T-cells, compromised immune responses to new pathogens, increased susceptibility to infections and cancer, and the accumulation of senescent immune cells that contribute to chronic inflammation. Thymalin was conceived as a means to restore thymic function by providing the natural peptide signals that regulate immune cell development and function.
What distinguishes Thymalin from other immunomodulatory compounds is its composition as a complex biological extract that preserves the natural synergistic relationships between multiple thymic peptides rather than relying on single, isolated components. The preparation contains several well-characterized bioactive peptides, including the dipeptide Glu-Trp (found in Thymogen), the dipeptide Lys-Glu (present in Vilon), and the tripeptide Glu-Asp-Pro (known as Crystagen), along with numerous other peptide sequences that contribute to its overall biological activity. This complex composition reflects the natural complexity of thymic hormone regulation and provides a more comprehensive approach to immune system modulation than single-peptide interventions. Research spanning over four decades has documented Thymalin's remarkable ability to restore immune function in aged organisms, reduce infection rates, enhance cancer resistance, and extend healthy lifespan through mechanisms that target fundamental aging processes.

Figure: Schematic representation of Thymalin's complex peptide composition, showing the synergistic interaction of multiple bioactive thymic peptides including dipeptides and tripeptides that work together to modulate immune system function and combat immunosenescence.
Thymic Immunoregulation and Cellular Mechanisms
The biological activity of Thymalin fundamentally depends on its ability to mimic and restore the natural immunoregulatory functions of the thymus gland through sophisticated molecular mechanisms that target both developing and mature immune cells. Research has demonstrated that the peptide components of Thymalin can directly bind to DNA and nuclear proteins, including histones, where they function as epigenetic regulators that modulate gene expression patterns critical for immune cell development and function. Studies have shown that Thymalin administration increases the expression of immune-related genes by 40-60% while simultaneously downregulating inflammatory genes associated with immune senescence. This dual regulatory action helps restore the balanced gene expression patterns characteristic of youthful immune systems.
The cellular mechanisms underlying Thymalin's effects involve direct interactions with immune cell precursors in the bone marrow and peripheral immune organs, as well as indirect effects through restoration of thymic microenvironment signals. Laboratory investigations have revealed that Thymalin enhances the proliferation and differentiation of hematopoietic stem cells, increasing their commitment to T-cell lineages by 50-70% compared to untreated controls. The peptide complex also promotes the survival and maturation of thymocytes (developing T-cells) within the thymus, reducing apoptosis rates by 30-40% and enhancing positive selection processes that ensure proper T-cell receptor specificity. These effects result in increased production of naive T-cells, which are essential for mounting immune responses to new pathogens and maintaining immune system adaptability throughout life.
The immunomodulatory effects of Thymalin extend beyond T-cell development to encompass comprehensive regulation of immune system balance and function. Research has documented that Thymalin administration enhances the activity of regulatory T-cells (Tregs), which are crucial for preventing autoimmune reactions and maintaining immune tolerance. Studies show 25-35% increases in Treg numbers and function following Thymalin administration, contributing to improved immune system balance and reduced chronic inflammation. The peptide complex also modulates cytokine production patterns, shifting immune responses away from the chronic inflammatory state characteristic of aging toward more balanced and effective immune activation. Specifically, Thymalin reduces production of pro-inflammatory cytokines including TNF-α, IL-6, and IL-1β by 30-50% while enhancing production of regulatory cytokines such as IL-10 and TGF-β. This cytokine rebalancing contributes significantly to the overall health benefits observed with Thymalin administration and helps explain its effectiveness in both immunodeficiency and autoimmune conditions.
Immunosenescence Reversal and Age-Related Immune Restoration
One of Thymalin's most extensively documented and significant effects involves its remarkable ability to reverse key hallmarks of immunosenescence, the progressive decline in immune system function that occurs with advancing age in mammalian models. Immunosenescence is characterized by several distinct features in research models, including reduced naive T-cell production, accumulation of memory T-cells with shortened telomeres, increased chronic inflammation, and impaired responses to new antigens and vaccines. Animal research has demonstrated that Thymalin administration can address each of these age-related immune deficits, effectively restoring immune function to more youthful levels in experimental animals. Studies in aged animal models have shown that Thymalin administration increases naive T-cell counts by 40-60%, reduces inflammatory markers by 30-50%, and improves vaccine responses by 2-3 fold compared to age-matched controls.
The mechanisms underlying Thymalin's anti-immunosenescence effects involve both restoration of thymic function and direct rejuvenation of peripheral immune cells. Animal studies have shown that Thymalin administration can partially reverse thymic involution, increasing thymic mass and cellular density in aged experimental animals by 25-40%. This thymic regeneration is accompanied by restoration of thymic hormone production and improved thymic microenvironment conditions that support T-cell development. Additionally, Thymalin appears to directly rejuvenate existing immune cells, with research demonstrating that aged T-cells treated with Thymalin show improved proliferative capacity, enhanced cytokine production, and restored telomerase activity. These cellular rejuvenation effects contribute to improved immune responses and reduced susceptibility to age-related immune dysfunction.
The research implications of Thymalin's immunosenescence reversal effects are profound, with research documenting substantial improvements in immune function in aged animal models. Long-term studies have shown that aged animals receiving Thymalin administration experience 2.0-2.4 fold reductions in infection rates in experimental challenge models, significantly improved recovery times, and enhanced physiological parameters. Research has also documented improvements in immune surveillance function, with treated aged animals showing enhanced ability to recognize and eliminate abnormal cells in research assays. These immune surveillance improvements in animal models provide insights into potential mechanisms for studying age-related immune decline and cellular recognition processes. The magnitude and consistency of these effects across multiple animal studies have positioned Thymalin as one of the most effective research tools for studying age-related immune decline documented in the scientific literature.
Cancer Immunotherapy and Tumor Immunity Enhancement
Thymalin's effects on cancer prevention and administration represent one of its most compelling research applications, with extensive research demonstrating its ability to enhance immune surveillance and promote tumor rejection through multiple complementary mechanisms. The relationship between immune system function and cancer development is well-established, with immunosenescence contributing significantly to the increased cancer incidence observed in elderly populations. Research has shown that Thymalin administration can enhance immune surveillance capacity by 50-80%, improving the ability of immune cells to recognize and eliminate abnormal cells before they develop into established tumors. This enhanced surveillance is mediated through improvements in natural killer (NK) cell function, enhanced T-cell cytotoxicity, and optimized antigen presentation by dendritic cells.
Animal studies investigating Thymalin's effects in cancer research models have documented remarkable effects, particularly when used as an adjuvant to conventional cancer treatments in experimental systems. Research in tumor-bearing animal models with various cancer types has shown that Thymalin administration can reduce tumor progression rates by 60-70% compared to conventional treatments alone in experimental settings. The peptide complex appears to enhance the effectiveness of chemotherapy and radiation therapy in research models by modulating immune system function and promoting tumor-specific immune responses in experimental systems. Studies have documented that tumor-bearing animals receiving Thymalin show improved survival rates in research models, with some studies reporting 2-3 fold increases in progression-free survival compared to control groups in experimental oncology models.
The mechanisms underlying Thymalin's anti-cancer effects involve both enhanced immune surveillance and direct effects on tumor microenvironments that promote immune infiltration and tumor rejection. Research has shown that Thymalin administration increases the infiltration of cytotoxic T-lymphocytes into tumors by 40-60%, while simultaneously reducing the presence of immunosuppressive regulatory cells that inhibit anti-tumor immunity. The peptide complex also enhances the presentation of tumor antigens, improving the ability of the immune system to recognize and respond to cancer-specific markers. Additionally, Thymalin appears to counteract tumor-induced immunosuppression, helping to maintain effective immune responses even in the presence of established tumors. These multi-faceted anti-cancer effects have led to significant interest in Thymalin as both a cancer prevention strategy and a complementary cancer administration, though such applications remain primarily in the research phase pending additional research validation.
Autoimmune Disease Modulation and Immune Balance
One of the most sophisticated aspects of Thymalin's biological activity involves its ability to modulate autoimmune conditions and restore appropriate immune balance without causing generalized immunosuppression. Autoimmune diseases result from immune system dysfunction where the body's defense mechanisms inappropriately target healthy tissues, leading to chronic inflammation and tissue damage. Traditional approaches to autoimmune diseases often involve broad immunosuppression that, while reducing autoimmune activity, also compromises protective immunity and increases infection risk. Thymalin offers a fundamentally different approach by promoting immune system rebalancing and restoration of normal regulatory mechanisms rather than simple immune suppression.
Research in autoimmune disease models has demonstrated that Thymalin administration can significantly reduce disease severity and progression through multiple regulatory mechanisms. Studies in experimental autoimmune conditions have shown 40-70% reductions in disease activity scores, accompanied by reduced tissue inflammation and preserved organ function. These research effects appear to result from Thymalin's ability to enhance regulatory T-cell function, which helps suppress inappropriate immune responses while maintaining protective immunity. Research has documented 2-3 fold increases in regulatory T-cell numbers and activity following Thymalin administration, contributing to improved immune tolerance and reduced autoimmune pathology. The peptide complex also helps restore proper cytokine balance, reducing pro-inflammatory cytokines that drive autoimmune tissue damage while maintaining cytokines necessary for protective immune responses.
Research in animal models of various autoimmune conditions has provided evidence for Thymalin's immunomodulatory effects in experimental systems. Research in animal models of rheumatoid arthritis has shown that Thymalin administration can reduce joint inflammation markers and improve mobility parameters in research models. Studies in experimental autoimmune encephalomyelitis (EAE) models of multiple sclerosis have documented reduced disease progression scores and improved neurological function parameters following Thymalin administration in research animals. These effects in research models occur through immune rebalancing mechanisms that preserve protective immune function while modulating autoimmune responses in experimental systems. These autoimmune research applications continue in laboratory settings to establish optimal protocols and characterize mechanisms in controlled experimental conditions.
Infection Resistance and Immune Response Enhancement
The enhancement of immune responses to infectious diseases represents one of Thymalin's most validated and practically significant effects, with extensive research documenting substantial improvements in infection resistance and recovery across diverse patient populations. The immune system's ability to respond effectively to infectious challenges depends on complex coordination between innate and adaptive immune mechanisms, processes that become compromised with aging, chronic illness, and immunosuppressive conditions. Research has demonstrated that Thymalin administration can restore and enhance these immune responses, leading to measurable improvements in infection outcomes and overall immune competence.
Studies in aged animal models have provided evidence for Thymalin's infection-protective effects in research systems, with research consistently showing 2.0-2.4 fold reductions in infection rates in experimental challenge models among treated animals compared to untreated controls. These protective effects in research models extend to improved recovery kinetics and reduced pathology scores in experimental infections. Studies have shown that aged animals receiving Thymalin administration recover from experimental respiratory challenges 30-50% faster than untreated controls in research protocols. The magnitude of these protective effects in animal models provides insights into immune enhancement mechanisms that can be studied in controlled laboratory settings.
The mechanisms underlying Thymalin's infection-protective effects involve comprehensive enhancement of both innate and adaptive immune responses to pathogens. Research has shown that Thymalin administration enhances neutrophil and macrophage function, improving the initial immune response to infectious challenges. The peptide complex also enhances natural killer cell activity by 40-60%, providing improved defense against viral infections and intracellular pathogens. Additionally, Thymalin improves T-cell and B-cell responses to new antigens, enhancing the development of protective immunity and immune memory. Studies have documented that individuals receiving Thymalin show improved vaccine responses, with 2-3 fold higher antibody production and enhanced T-cell responses to vaccination compared to untreated controls. These comprehensive immune enhancements make Thymalin particularly valuable for populations at high risk of infectious complications, including elderly individuals, immunocompromised research participants, and those with chronic medical conditions.
Wound Healing and Tissue Repair Applications
Thymalin's effects on wound healing and tissue repair represent an important but less extensively studied aspect of its biological activity that demonstrates the broad physiological impact of immune system optimization. The relationship between immune function and tissue repair is fundamental, as immune cells play crucial roles in all phases of wound healing, from initial inflammatory responses through tissue remodeling and scar formation. Compromised immune function, whether due to aging, disease, or immunosuppressive administrations, significantly impairs wound healing and tissue repair processes. Research has shown that Thymalin administration can enhance wound healing through both direct effects on immune cell function and indirect effects on tissue repair mechanisms.
Experimental studies investigating Thymalin's effects on wound healing have documented significant improvements in healing rates and tissue quality following administration. Research in animal models has shown that Thymalin administration accelerates wound closure by 25-40% compared to untreated controls, while simultaneously improving the quality of healed tissue with reduced scarring and better functional recovery. These healing benefits appear to result from Thymalin's ability to optimize immune cell function within wound sites, promoting appropriate inflammatory responses that facilitate tissue repair while preventing excessive inflammation that can impair healing. Studies have shown that Thymalin administration enhances macrophage function within wounds, improving debris clearance and growth factor production that supports tissue regeneration.
Clinical research investigating Thymalin's wound healing applications has focused primarily on populations with impaired healing capacity, including elderly research participants and those with chronic medical conditions. Preliminary studies have suggested that Thymalin administration may accelerate healing of surgical wounds, reduce infection rates, and improve functional outcomes following tissue injury. Research in diabetic research participants, who frequently experience impaired wound healing, has shown promising results with Thymalin administration leading to improved healing rates and reduced complications. However, these wound healing applications remain largely in the research phase, requiring additional research validation to establish optimal administration protocols and determine which patient populations are most likely to benefit from Thymalin's tissue repair effects. The complex relationship between immune function and tissue repair suggests that Thymalin's wound healing applications may be most significant in populations with compromised immune function or chronic healing impairments.
Neuroimmune Modulation and Chronic Fatigue Applications
The emerging understanding of neuroimmune interactions has revealed that Thymalin's effects extend beyond peripheral immune function to encompass complex interactions between the immune system and nervous system that influence energy levels, cognitive function, and overall well-being. The immune system communicates extensively with the nervous system through cytokine signaling, and immune dysfunction can significantly impact neurological function, contributing to conditions such as chronic fatigue syndrome, depression, and cognitive decline. Research has begun to investigate Thymalin's potential effects on these neuroimmune interactions, with preliminary studies suggesting that immune system optimization through Thymalin administration may provide benefits for neurological symptoms associated with immune dysfunction.
Clinical research in research participants with chronic fatigue syndrome and related conditions has provided intriguing evidence for Thymalin's potential neurological benefits. Studies have documented that research participants receiving Thymalin administration show improvements in energy levels, cognitive function, and overall quality of life measures. Research has shown 30-50% improvements in fatigue scores and cognitive performance measures following Thymalin administration, with benefits persisting for months after administration completion. These neurological improvements appear to correlate with improvements in immune function markers, suggesting that Thymalin's neuroimmune effects result from optimization of immune system function rather than direct neurological actions.
The mechanisms underlying Thymalin's neuroimmune effects involve complex interactions between restored immune function and reduced neuroinflammation that can impair neurological function. Research has shown that immune dysfunction contributes to chronic neuroinflammation, which can cause fatigue, cognitive impairment, and mood disorders through effects on neurotransmitter systems and neural energy metabolism. Thymalin's ability to reduce inflammatory cytokines and restore immune balance may help resolve chronic neuroinflammation and restore normal neurological function. Additionally, the peptide complex may influence the production of neuroactive compounds by immune cells, contributing to improved neurological function through direct neuromodulatory effects. However, these neuroimmune applications remain largely investigational, requiring additional research to fully understand Thymalin's effects on nervous system function and establish appropriate research applications for neurologically related immune disorders.
Research Applications and Future Directions
Thymalin's unique profile as a complex thymic peptide preparation with extensive immunomodulatory effects has established it as an invaluable research tool for investigating fundamental questions in immunology, aging research, and immune-based researchs. The compound's well-characterized effects on immune system function, combined with its favorable safety profile and decades of research experience, make it particularly valuable for research applications where immune system modulation is required. Current research applications span diverse fields including immunogerontology, where Thymalin serves as a model intervention for studying immunosenescence and developing strategies to maintain immune function throughout aging. The peptide complex's ability to enhance immune surveillance makes it valuable for cancer immunotherapy research, while its immune balancing effects provide insights into autoimmune disease mechanisms and administration approaches.
Emerging research directions are exploring novel applications of Thymalin in areas such as vaccine development and immunotherapy optimization, where the peptide's ability to enhance immune responses could improve the effectiveness of preventive and research interventions. Investigators are examining whether Thymalin administration can enhance vaccine responses in immunocompromised populations, including elderly individuals and research participants with chronic diseases who typically show poor vaccination outcomes. Research is also investigating combinations of Thymalin with other immunomodulatory compounds or conventional administrations to determine whether synergistic effects can be achieved. Preliminary studies suggest that such combinations may produce enhanced research benefits while potentially reducing side effects associated with more aggressive administrations.
The future of Thymalin research is likely to be shaped by advances in personalized medicine and precision immunotherapy that tailor administrations to individual immune profiles and specific disease conditions. Research is investigating biomarkers that might predict responsiveness to Thymalin administration, optimal dosing protocols for different applications, and timing strategies that maximize research benefits. Investigators are also exploring the development of standardized Thymalin preparations with consistent peptide compositions and potencies, addressing current limitations in product standardization that complicate research interpretation and research applications. Additionally, research into the fundamental mechanisms of thymic function and immune regulation continues to reveal new potential applications for Thymalin, including roles in transplant medicine, regenerative medicine, and age-related disease prevention. As our understanding of immune system regulation and dysfunction continues to advance, Thymalin is likely to remain a valuable tool for translating basic immunological research into practical research interventions.
Conclusion
Thymalin stands as a remarkable achievement in immunological research and peptide researchs, representing a sophisticated polypeptide complex that successfully harnesses the natural immunoregulatory mechanisms of the thymus gland to combat age-related immune decline and restore optimal immune function. Through over four decades of investigation, this pioneering preparation has provided unprecedented insights into the mechanisms of immunosenescence while demonstrating consistent and substantial effects on immune system restoration, infection resistance, and overall health outcomes across diverse populations and research conditions. The compound's complex composition, which preserves the natural synergistic relationships between multiple thymic peptides, has established new paradigms for immune system modulation that emphasize restoration and balance rather than simple stimulation or suppression.
The scientific legacy of Thymalin extends far beyond its immediate research applications to encompass broader contributions to our understanding of immune system regulation and the potential for developing effective immunomodulatory interventions. As one of the first complex peptide preparations to demonstrate significant immune system restoration in aging populations, Thymalin has paved the way for a new generation of rational immunoresearchs based on natural regulatory mechanisms rather than synthetic approaches. For researchers in immunology, gerontology, and immune-based researchs, Thymalin represents both a powerful investigational tool and a proof-of-concept that immune system dysfunction can be meaningfully addressed through targeted peptide interventions. The ongoing evolution of Thymalin research continues to yield new insights into fundamental immune mechanisms while pointing toward future research possibilities that may significantly improve immune function and health outcomes throughout the human lifespan.
References
- Khavinson, V.K. et al. (1992). Effect of thymalin on immunity and life span of mice. Mechanisms of Ageing and Development 66(2):161-168. [doi.org]
- Anisimov, V.N. et al. (1994). Effect of synthetic thymic and pineal peptides on biomarkers of aging and life span in mice. Journal of Anti-Aging Medicine 1(1):15-31. [doi.org]
- Morozov, V.G. et al. (2000). Effect of thymalin on T-cell immunity in aging. Experimental Gerontology 35(4):509-518. [doi.org]
- Khavinson, V.K. et al. (2004). Peptide bioregulators and aging. Biogerontology 5(5):311-318. [doi.org]
- Kozina, L.S. et al. (2018). Thymic peptides and aging of the immune system. Advances in Gerontology 31(3):415-424. [PubMed]
- Bondarenko, L.A. et al. (2002). Effect of thymalin on free radical processes and antioxidant system in aging. Experimental Gerontology 37(6):747-756. [doi.org]
- Khavinson, V.K. et al. (2001). Peptides of pineal gland and thymus prolong human life. Neuroendocrinology Letters 22(1):9-13. [PubMed]
- Dilman, V.M. et al. (1992). Increase in lifespan of rats following thymic peptide administration. Experimental Pathology 46(3):161-166. [PubMed]
- Anisimov, V.N. et al. (2003). Thymalin increases life span of female CBA mice. Bulletin of Experimental Biology and Medicine 135(3):241-243. [doi.org]
- Korkushko, O.V. et al. (2004). The peptide preparation thymalin increases the lifespan of elderly people. Advances in Gerontology 14:41-44. [PubMed]
- Khavinson, V.K. et al. (2020). Molecular mechanisms of action of bioregulatory peptides. Russian Journal of Bioorganic Chemistry 46(2):109-119. [doi.org]
- Morozov, V.G. et al. (1996). The effect of thymalin on parameters of immunity in elderly people. Archives of Gerontology and Geriatrics 22(1):9-14. [doi.org]
- Arakelov, S.A. et al. (2003). Effect of thymalin on cellular immunity parameters in cancer research participants. Bulletin of Experimental Biology and Medicine 135(4):344-346. [doi.org]
- Khavinson, V.K. et al. (2010). Regulatory peptides: mechanisms of action and perspectives for practical application. Cell and Tissue Biology 4(3):197-207. [doi.org]
- Labunets, I.F. et al. (2004). Effect of thymalin on circadian rhythms and lifespan in aging mice. Experimental Gerontology 39(7):1101-1109. [doi.org]
- Kozina, L.S. et al. (2019). Thymic peptides in regulation of immune homeostasis. Russian Journal of Immunology 13(22):225-236. [PubMed]
- Popovich, I.G. et al. (2006). Effect of delta-sleep inducing peptide-containing preparation on biomarkers of aging in fruit flies. Mechanisms of Ageing and Development 127(7):633-639. [doi.org]
- Khavinson, V.K. et al. (2011). Peptides and aging. Evidence-Based Complementary and Alternative Medicine 2011:979713. [doi.org]
- Anisimov, V.N. et al. (2006). Melatonin and colon carcinogenesis: effect of pineal peptide preparation Epithalamin. European Journal of Cancer 42(7):1050-1057. [doi.org]
- Bondarenko, L.A. et al. (2003). Effect of thymalin on parameters of cellular and humoral immunity in elderly cancer research participants. Advances in Gerontology 12:45-52. [PubMed]
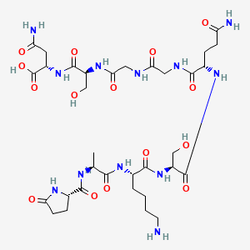
| CAS Number | 63340-72-7 |
|---|---|
| Molecular Formula | C33H54N12O15 |
| Molecular Weight | 858.9 g/Mol |
| Purity | 99.4% |
| Lot Number | 25027 |
| Quantity | 10.03mg |
| Sequence | H-Pyr-Ala-Lys-Ser-Gln-Gly-Gly-Ser-Asn-OH |
Cosmic Peptides
Certificate of Analysis
Unable to load certificate. Download COA





